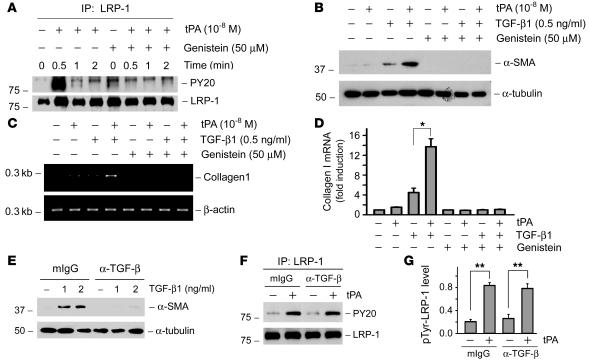
Figure 4. Tyrosine phosphorylation of LRP-1 is required for mediating the fibrogenic action of tPA and is independent of TGF-β signaling.
(A) tPA induced a rapid tyrosine phosphorylation of LRP-1 on its β subunit, and genistein abolished tPA-triggered LRP-1 phosphorylation. Cell lysates after various treatments were immunoprecipitated with anti–LRP-1 antibody, followed by immunoblotting with anti-phosphotyrosine antibody (PY20). (B–D) Inhibition of tyrosine phosphorylation of LRP-1 by genistein abolished the effects of tPA on α-SMA (B) and type I collagen (C and D) induction. Quantitative determination of type I collagen mRNA levels in different groups is presented in D. *P < 0.05. (E) Monoclonal pan-specific TGF-β–neutralizing antibody (α–TGF-β) (25 μg/ml) blocked the α-SMA expression induced by TGF-β1 in NRK-49F cells. Same amount of normal mouse IgG (mIgG) was used as negative controls. (F and G) Blockade of TGF-β signaling with neutralizing antibody did not affect the tPA-induced LRP-1 tyrosine phosphorylation. Representative western blot (F) and graphic presentation of the relative phosphorylated LRP-1 levels (G) are presented. **P < 0.01.