Abstract
Neonatal hepatitis and biliary hypoplasia are not recognised features of Williams syndrome. A case of Williams syndrome, presenting with neonatal conjugated hyperbilirubinaemia leading to an initial misdiagnosis is reported.
Keywords: biliary hypoplasia, Williams syndrome, conjugated hyperbilirubinaemia, Alagille syndrome
The child (RS) was born at term by spontaneous vertex delivery with a birth weight of 2580 g (9th centile). He presented at the age of 6 weeks with poor feeding. His weight was 3520 g (2nd–9th centile). He was jaundiced and had a grade 3/6 systolic murmur which was loudest in the pulmonary area. Liver function tests showed a conjugated hyperbilirubinaemia and raised transaminases (table 1).
Table 1 Liver function and calcium levels.
| Age (weeks) | 6 | 8 | 17 | 22 | 45 | 57 | Reference range |
|---|---|---|---|---|---|---|---|
| Total bilirubin (μmol/l) | 229 | 257 | 109 | 20 | 6 | <5 | 0–20 μmol/l |
| Conjugated bilirubin (μmol/l) | 185 | 226 | 87 | NA | <5 | <5 | 0–20 μmol/l |
| AST (U/l) | 178 | 473 | 148 | 581 | 134 | 66 | 15–45 U/l |
| ALT (U/l) | 185 | 305 | 158 | 774 | 120 | 69 | 10–40 U/l |
| Serum calcium (mmol/l) | 2.58 | 2.68 | 2.83 | 2.53 | 3.81 | 2.75 | 2.20–2.70 mmol/l |
| Serum albumin (g/l) | 35 | 37 | 39 | 39 | 47 | 40 | 30–45 g/l |
NA, not available.
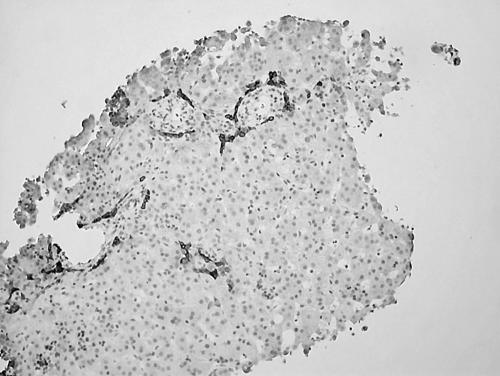
Investigations for neonatal hepatitis, including α1 antitrypsin phenotype were normal. An HIDA scan showed normal uptake in the liver but no evidence of tracer in the gallbladder and negligible excretion into the gut, raising the possibility of biliary atresia. Liver biopsy showed bile stasis, dilated sinusoids, and a paucity of intrahepatic bile ducts (fig 1). An echocardiogram showed bilateral pulmonary artery branch obstruction, mild supravalvar aortic obstruction, and a small atrial septal defect.
Figure 1 Liver biopsy (AE1/AE3 stain) showing biliary hypoplasia with epithelial cell recruitment at margins of portal tract.
A diagnosis of Alagille syndrome was made on the basis of the liver biopsy findings, chronic cholestasis, and peripheral pulmonary stenosis; a clinical geneticist felt that the facial features were compatible with this diagnosis.1 There was, however, no evidence of posterior embryotoxon on ophthalmolological examination and no vertebral abnormalities on x ray examination. No mutation was identified in JAG1.2
The conjugated hyperbilirubinaemia gradually resolved over a period of six months (table 1) and the transaminases improved, although did not return to the normal range.
At the age of 10 months he presented with vomiting, fever, irritability, lethargy, and constipation. He was hypertensive (117/73 mm Hg) and overtly hypercalcaemic with a serum calcium level of 3.81 mmol/l.
RS now had more obvious facial features suggesting a diagnosis of Williams syndrome.3 He was a small child with developmental delay who was unable to sit up at the age of 10 months. He now also had a number of serum calcium estimations which were borderline high (table 1). A FISH test confirmed the diagnosis of Williams syndrome with a deletion detected at the elastin gene locus 7q11.23.
RS continues to be followed up as an outpatient. His calcium levels remain stable on low calcium milk (Locasol). His bilirubin levels are normal but transaminases remain mildly elevated. A repeat echocardiogram showed moderate right pulmonary artery stenosis but no supravalvar aortic stenosis.
Discussion
Conjugated hyperbilirubinaemia and biliary hypoplasia are not known to be features of Williams syndrome.
In this case, the initial presenting features were those of prolonged jaundice, biliary hypoplasia, and pulmonary branch stenosis, leading to the incorrect diagnosis of Alagille syndrome. Our attention has since been brought to other cases of Williams syndrome and prolonged conjugated hyperbilirubinaemia, one of whom was also initially thought to have Alagille syndrome (Metcalfe K; personal communication, 2005). There is some overlap between the clinical features of Alagille syndrome and Williams syndrome.3 Both syndromes are associated with poor feeding, short stature, and developmental delay. Cardiac abnormalities occur in both syndromes, and although classically Williams syndrome is associated with supravalvar aortic stenosis, pulmonary branch stenosis is also common.3,5 It has been suggested that Williams syndrome should be looked for in all cases of supravalvar aortic stenosis or pulmonary branch stenosis.6 Although some minor dysmorphic features are shared by both syndromes, for example, depressed nasal bridge, the facies are described as being quite different. However, we highlight the difficulty in detecting subtle features in a baby of 6 weeks of age. In our patient the facial characteristics were much more apparent at the age of 10 months.
Mutations in the JAG1 gene on chromosome 20p12 are thought to be present in 70% of cases of Alagille syndrome,2 but testing for mutations in the JAG1 gene is not available at all centres. The diagnosis of Alagille syndrome is often a clinical one. The FISH test for Williams syndrome is more easily accessible and shows an elastin gene deletion in over 96% of cases.4
In conclusion, there are clinical similarities between Williams syndrome and Alagille syndrome. In our case the presence of conjugated hyperbilirubinaemia and biliary hypoplasia, not documented as features of Williams syndrome, led to the misdiagnosis of Alagille syndrome. If we had adopted a policy of FISH test for Williams syndrome in all cases of supravalvar aortic stenosis or pulmonary branch stenosis, the correct diagnosis would have been established earlier. We also emphasise the importance of being aware of the calcium levels in cases of presumed Alagille syndrome, particularly those in which not all clinical features are present. In addition, our report of biliary hypoplasia in this case further extends the phenotype of Williams syndrome.
Abbreviations
AST - aspartate aminotransferase
ALT - alanine aminotransferase
HIDA - hepatobiliary iminodiacetic acid
FISH - fluorescent in‐situ hybridisation
Footnotes
Competing interests: none declared
References
- 1.Alagille D, Estrada A, Hadchouel M.et al Syndromic paucity of interlobular bile ducts. J Pediatr 1987110195–200. [DOI] [PubMed] [Google Scholar]
- 2.Krantz I, Piccoli D, Spinner N. Clinical and molecular genetics of Alagille syndrome. Curr Opin Pediatr 199911558–564. [DOI] [PubMed] [Google Scholar]
- 3.Winter R M, Baraitser M.London dysmorphology database, version 3.0. Oxford: Oxford Medical Databases, Oxford University Press, 2001
- 4.Lowery M C, Morris C A, Ewawrt A.et al Strong correlation of elastin deletions detected by FISH with Williams syndrome: evaluation of 235 patients. Am J Hum Genet 19955749–53. [PMC free article] [PubMed] [Google Scholar]
- 5.Eronen M, Peippo M, Hiippala A.et al Cardiovascular manifestations in 75 patients with Williams syndrome. J Med Genet 200239554–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.St Heaps L, Robson L, Smith A. Review of referrals for the FISH detection of Williams syndrome highlights the importance of testing in supravalvular aortic stenosis/pulmonary stenosis. Am J Med Genet 200198109–111. [DOI] [PubMed] [Google Scholar]