Abstract
Background
Evidence of premature atherosclerosis and systemic arterial stiffening in patients after Kawasaki disease is accumulating.
Aim
To test the hypothesis that carotid intima‐media thickness (IMT), a surrogate marker of atherosclerosis, is associated with systemic arterial stiffness in children after Kawasaki disease.
Methods
A cohort of 72 patients was studied, comprising 26 patients with Kawasaki disease and coronary aneurysms (group I), 24 patients with Kawasaki disease and normal coronary arteries (group II) and 22 healthy age‐matched children (group III). The carotid IMT, carotid artery stiffness index, brachioradial pulse wave velocity (PWV), fasting total cholesterol, high‐density lipoprotein (HDL) cholesterol and low‐density lipoprotein (LDL) cholesterol were determined and compared among the three groups.
Results
The carotid IMT was related to indices of arterial stiffness, and significant determinants of carotid IMT were identified by multivariate analysis. The mean (standard deviation (SD)) carotid IMT of both group I (0.41 (0.04) mm) and group II (0.39 (0.04) mm) was significantly greater than that of group III (0.36 (0.04) mm; p<0.001 and p = 0.008, respectively). For the entire cohort, carotid IMT correlated positively with LDL cholesterol (r = 0.31, p = 0.009), carotid artery stiffness index (r = 0.40, p = 0.001) and brachioradial PWV (r = 0.28, p = 0.016), but not with age, body mass index, systemic blood pressure, and HDL and total cholesterol. Multiple linear regression analysis identified carotid artery stiffness index (β = 0.25, p = 0.028) and subject grouping (β = −0.39, p = 0.001; model R2 = 0.29) as significant correlates of carotid IMT.
Conclusion
The increased carotid IMT in children after Kawasaki disease is associated with systemic arterial stiffening.
There is increasing evidence to suggest that children with a history of Kawasaki disease might be predisposed to premature atherosclerosis.1,2,3,4 Endothelial dysfunction, a key event of atherosclerosis, has been seen in children after Kawasaki disease, regardless of their having coronary artery lesions.1 We and others have further shown the proatherogenic changes in the lipid profile2,3,4 of these patients. Noto et al5 found an increase in carotid intima‐media thickness (IMT), a surrogate marker of atherosclerosis, in both children6 and adults7 late after the acute disease in patients with coronary artery lesions. Stiffening of the central and peripheral arteries has also been found in patients with Kawasaki disease.4,5,8,9 Although traditionally regarded as part of the normal ageing process, arterial stiffening may precede clinical disease and predict a higher risk for developing atherosclerosis, hypertension and stroke in adults.10 Previous studies examining the association between arterial stiffness and atherosclerosis in high‐risk adults have shown a significant positive relationship.11,12,13,14 We hypothesised that carotid IMT is associated with systemic arterial stiffness in children after Kawasaki disease. Accordingly, the objective of our study was to test this hypothesis by examining the association between carotid IMT, a marker of atherosclerosis, and indices of carotid and brachioradial artery stiffness in these patients.
Methods
Participants
Patients with a history of Kawasaki disease were recruited from the Paediatric Cardiac Clinic, Grantham Hospital, Hong Kong, China. All recruited patients had normal left ventricular shortening fraction and absence of valvar incompetence as documented by serial echocardiographic studies. Healthy age‐matched participants, who were children previously discharged from our clinic with a diagnosis of functional heart murmur and their healthy siblings, were recruited as controls. The institutional ethics committee approved the study and parents of all participants gave written, informed consent.
We reviewed the case records of the patients and collected the following data: interval from disease onset to time of study, coronary artery lesions, cardiac symptoms and drugs at the time of study. Coronary aneurysms were documented by serial two‐dimensional echocardiography. The participants were categorised into three groups for comparisons: group I, patients with persistent or regressed coronary aneurysms; group II, patients who never had echocardiographically documented coronary artery lesions; and group III, healthy controls.
The participants were examined after an overnight fast. The body weight and height were measured, and the body mass index (weight (kg)/height2 (m2) was calculated accordingly. Blood pressure in the right arm was measured twice using an automatic oscillometric device (Dinamap, Critikon, Tampa, Florida, USA), with the participants in the seated position after at least 10 min of rest. The average of the two blood pressure readings was taken.
Assessment of arterial stiffness
The carotid artery stiffness was assessed by calculating the stiffness index as reported previously.15,16 Briefly, a 7–15 MHz linear‐array transducer, interfaced to a Hewlett‐Packard Sonos 5500 ultrasound machine (Hewlett‐Packard Corp, Andover, MA, USA), was used to image the right carotid artery at about 1 cm proximal to the carotid bifurcation. The systolic and diastolic diameters were measured between the intima of the near and far walls. Three measurements each of systolic and diastolic diameters were averaged for the calculation of stiffness index according to the formula:
ln (SBP/DBP)/(δD/D),
where ln denotes natural logarithm, SBP systolic blood pressure, DBP diastolic blood pressure, δD the difference between systolic and diastolic diameters, and D the mean diameter.
The brachioradial artery stiffness was assessed by measuring the pulse‐wave velocity (PWV), using the previously reported photoplethysmographic technique.17 PWV is related to the square root of elastic modulus according to the Moens–Korteweg equation.18 Hence, the stiffer the artery, the faster the PWV. The transit time required for the pulse to travel from the brachial artery at the elbow to the radial artery at the wrist was determined from the time delay between the foot of the corresponding brachial and radial pulse waves. The average of three readings was taken. PWV was calculated by dividing the surface distance between the two sites of pulse wave recording by the transit time.
Carotid IMT
The right carotid artery was similarly imaged using the linear array transducer as aforementioned at about 1 cm proximal to the carotid bifurcation. The IMT of the common carotid artery far wall was measured using the electronic callipers of the ultrasound machine as described previously.5 All the measurements were carried out by a single investigator (SJW), blinded to the status of the participants and the stiffness indices. The average of three measurements was used for subsequent analyses.
Blood investigations
The fasting total cholesterol, high‐density lipoprotein (HDL) cholesterol and low‐density lipoprotein (LDL) cholesterol levels were determined. Plasma total cholesterol level was determined enzymatically using a Hitachi 912 analyzer (Roche Diagnostics, GmbH, Mannheim, Germany). HDL cholesterol was measured using a homogeneous method with polyethylene glycol‐modified enzymes and sulphated α‐cyclodextrin, and LDL cholesterol was calculated by the Friedewald equation.
Data analysis
Data are presented as mean (SD). One‐way analysis of variance was used to compare differences in the variables among the three groups, with retrospective comparisons using the Bonferroni test. For categorical variables, χ2 test was carried out. Pearson's correlation analysis was used to assess for correlations between carotid IMT and demographic variables, systemic blood pressure and indices of arterial stiffness. Stepwise multiple linear regression was used to identify significant determinants of carotid IMT. Values of p<0.05 were considered to be significant. All statistical analyses were carried out using SPSS V.11.5.
Results
Participants
We studied a total of 72 participants. Group I comprised 26 patients with Kawasaki disease, half of whom had persistent coronary aneurysms, whereas group II comprised 24 patients with Kawasaki disease. None had symptoms of myocardial ischaemia and none required coronary artery interventions. All but five of the patients with Kawasaki disease had received intravenous immunoglobulin treatment during the acute phase of illness and 13 were maintained on long‐term oral low‐dose aspirin treatment. Group III comprised 22 age‐matched controls.
Table 1 summarises the demographic variables, systemic blood pressure and lipid profiles of the participants. The three groups did not differ markedly with regard to age at the time of study, sex distribution, body mass index, systemic blood pressure, total cholesterol level and HDL cholesterol level. However, group I patients had higher LDL cholesterol level, as reported previously.4
Table 1 Demographic data, blood pressure and lipid profiles of participants in the three groups.
| Group I | Group II | Group III | p Value | |
|---|---|---|---|---|
| (n = 26) | (n = 24) | (n = 22) | ||
| Age at study (years) | 8.6 (2.8) | 8.6 (3.3) | 9.5 (2.5) | 0.52 |
| Age at diagnosis of KD (years) | 1.5 (1.7) | 2.7 (2.6) | — | 0.10 |
| Interval from diagnosis to time of study (years) | 7.4 (3.5) | 5.8 (2.1) | — | 0.09 |
| Sex (male:female) | 17:9 | 16:8 | 14:8 | 0.98 |
| BMI (kg/m2) | 16.9 (2.7) | 15.8 (2.0) | 16.9 (3.7) | 0.34 |
| SBP (mm Hg) | 107 (14) | 107 (8) | 107 (11) | 0.99 |
| DBP (mm Hg) | 58 (9) | 58 (5) | 57 (7) | 0.97 |
| Total cholesterol (mmol/l) | 4.5 (0.9) | 4.4 (0.9) | 4.1 (0.7) | 0.22 |
| HDL cholesterol (mmol/l) | 1.4±(0.3) | 1.5 (0.3) | 1.5 (0.4) | 0.29 |
| LDL cholesterol (mmol/l) | 2.8 (0.8) | 2.6 (0.8) | 2.2 (0.6) | 0.02* |
BMI, body mass index; DBP, diastolic blood pressure; HDL, high‐density lipoprotein; KD, Kawasaki disease; LDL, low‐density lipoprotein; SBP, systolic blood pressure; –, no data available.
Values are mean (SD) unless otherwise specified.
*Significant p value.
Arterial stiffness
The carotid artery stiffness index (p = 0.004) and brachioradial PWV (p = 0.011) differed significantly among the three groups. The differences remained significant even after adjusting for the differences in LDL cholesterol among groups. The mean (SD) stiffness index of group I, II and III was 4.72 (1.20), 4.22 (0.64) and 3.77 (0.92), respectively. The respective mean (SD) brachioradial PWV was 7.22 (1.67), 6.73 (1.88), and 5.77 (1.25) m/s. Post‐hoc analyses showed that group I had a significantly greater stiffness index (p = 0.003) and faster brachioradial PWV (p = 0.009) than group III. Therefore, a dose–response relationship between the degree of coronary artery involvement and arterial stiffness indices was hence suggested.
Carotid IMT
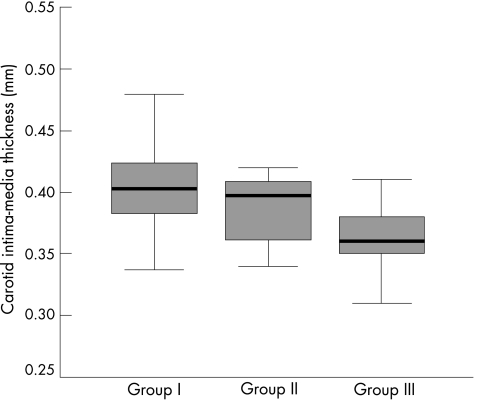
The carotid IMT similarly differed significantly among the three groups (p<0.001; fig 1). Post‐hoc analyses showed that the mean (SD) carotid IMT of group I (0.41 (0.04) mm, p<0.001) and group II (0.39 (0.04) mm, p = 0.008) was significantly greater than that of group III (0.36 (0.04) mm).
Figure 1 Box plots showing the distribution of carotid intima‐media thickness in the three groups of participants. The thick black line within each box represents the median in each group.
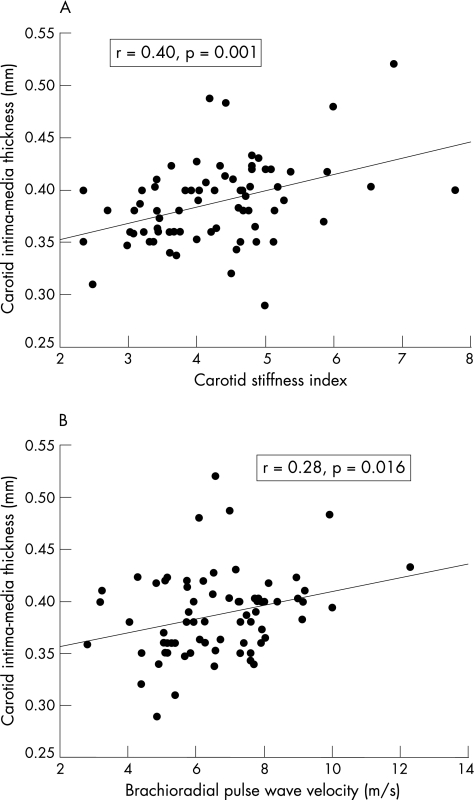
For the entire cohort, carotid IMT correlated positively with LDL cholesterol (r = 0.31, p = 0.009), carotid artery stiffness index (r = 0.40, p = 0.001) and brachioradial PWV (r = 0.28, p = 0.016; fig 2), but not with age, body mass index, systemic blood pressure, or HDL and total cholesterol. Multiple stepwise linear regression analysis of the entire cohort was used to identify significant correlates of carotid IMT. The dependent variables included were age, sex, body mass index, subject grouping, systolic and diastolic blood pressures, total cholesterol, HDL cholesterol, LDL cholesterol, carotid artery stiffness index and brachioradial PWV. Significant correlates were subject grouping (β = −0.39, p = 0.001) and carotid artery stiffness index (β = 0.25, p = 0.028; model R2 = 0.29). When only the patients (group I and II) were analysed, with addition of duration since onset of Kawasaki disease as a covariate into the multivariate model, total cholesterol level (β = 0.29, p = 0.03) and carotid artery stiffness index (β = 0.53, p<0.001) (model R2 = 0.35) were found to be significant.
Figure 2 Scatter plots showing a positive correlation between carotid intima–media thickness and (a) carotid stiffness index, and (b) brachioradial pulse‐wave velocity.
Discussion
Increase in carotid IMT has been reported in patients with Kawasaki disease and coronary artery lesions.5 Our study extends this finding to patients without documented coronary sequelae. Furthermore, to our knowledge, this is the first study to show a significant association between carotid IMT and systemic arterial stiffness in children after Kawasaki disease. After adjustment of the potential confounding influence of age, sex, systemic blood pressure and serum cholesterol levels, the significant correlates of carotid IMT in patients with Kawasaki disease are carotid artery stiffness index and total cholesterol level.
Carotid IMT has been regarded as a valid surrogate marker of atherosclerosis in adults7 and is associated with cardiovascular events.19,20 In high‐risk children with obesity,21 hypercholesterolaemia,22 type 1 diabetes mellitus23 and a family history of premature myocardial infarction,24 carotid intima‐media thickening has likewise been seen. In children with coronary aneurysms complicating Kawasaki disease, Noto et al5 reported carotid intima–media thickening, although patients without coronary artery lesions were not included in their study. A recent Japanese study,25 however, reported no differences in carotid IMT among patients with varying severity of coronary artery lesions and between patients and controls. Nonetheless, these findings have to be interpreted with caution as the number of patients studied in each group is too small to provide adequate statistical power, and controls were significantly older than patients. As age‐dependent physiological thickening of the carotid IMT begins in childhood,26 a comparison of the findings with those of age‐matched controls is essential.
Our finding of carotid intima‐media thickening not only in patients with Kawasaki disease but also in those without coronary artery lesions is perhaps not surprising. Even in regressed coronary artery aneurysms and angiographically normal coronary arterial segments, long‐term functional abnormalities have been described.27 Systemic arterial endothelial dysfunction has also been shown years after Kawasaki disease, even in patients without coronary artery involvement.1 Fatal cases of Kawasaki disease, related to extensive fibrointimal thickening of the coronary arterial wall, occurring years after apparent resolution of vascular inflammation and in the absence of early detectable coronary artery abnormalities, have also been reported.28 Patients without coronary artery lesions do not, therefore, seem to be immune from the hypothesised risk of premature atherosclerosis.
Stiffening of the aorta,8 carotid artery5 and brachioradial artery4 have been reported in children after Kawasaki disease. Senzaki et al9 measured the aortic input impedance during cardiac catheterisation in patients with Kawasaki disease and found increased characteristic impedance, lower total peripheral arterial compliance and greater indices of arterial wave reflection, regardless of the persistence of coronary artery lesions. Their findings provide further evidence of central and peripheral arterial stiffening after Kawasaki disease. Although the underlying mechanisms of the disease remain speculative, arterial stiffening has been attributed to structural changes and the subsequent reparative process during the acute illness,29 and changes in the vascular tone secondary to endothelial dysfunction.1 Our group has further provided evidence of ongoing low‐grade inflammation late after the acute phase of Kawasaki disease, which may have a role in increasing systemic arterial stiffness.15
The finding of an association between increased carotid IMT and systemic arterial stiffness in this study is consistent with previous studies in animal models,30 postmortem specimens,11 adults with various levels of cardiovascular risk,14,31 patients with hypertension13 and adults with atheromatous plaques in the thoracic aorta owing to various pathologies.12 Results of other studies are nonetheless conflicting.32,33,34 The recent evidence suggesting that IMT may reflect atherosclerosis only beyond a certain level35 may perhaps partly explain the controversy. Alternatively, the association may not be as obvious in early changes as that in advanced atherosclerotic changes in the arterial wall.36 In young adults, the weak positive association becomes stronger in patients with a worse cardiovascular risk profile.32 The adverse cardiovascular risk profile in patients with Kawasaki disease may perhaps have some significance in this regard.
The coexistence of and association between a marker of atherosclerosis, the carotid IMT, and systemic arterial stiffness in this study may partly reflect common aetiological factors relating to Kawasaki disease. Although these may be independent processes that reflect two separate entities of vascular damage in Kawasaki disease, several lines of evidence favour the hypothesis of a self‐perpetuating process. Experimental studies in monkeys fed on an atherogenic diet,30 spontaneously hypercholesterolaemic rabbits,37 and LDL‐receptor deficient hypercholesterolaemic rabbits38 suggest that atherosclerosis leads to stiffening of the aorta. Stiffening of the arterial wall, on the other hand, may increase intraluminal stress secondary to an increase in pulse pressure,39 which may predispose to vascular damage and atherosclerosis. Increased pulse pressure has indeed been shown to augment atherogenesis in primate models.40 It is intriguing, therefore, to find that only local carotid artery stiffness, rather than brachioradial artery stiffness, is a significant correlate of carotid IMT by multivariate analysis. The possibility of establishing a feedback loop owing to structural and functional abnormalities of the arteries in patients with Kawasaki disease may be hypothesised, although further longitudinal studies are undoubtedly needed to determine the temporal sequence of carotid intima‐media thickening and arterial stiffening after Kawasaki disease.
Even in patients without coronary artery lesions, a proatherogenic lipid profile,4 endothelial dysfunction,1 systemic arterial stiffening4,9 and increased carotid IMT have been found. Ongoing counselling on cardiovascular risk factor reduction is therefore important. Periodic assessment of the cardiovascular risk factors every 3–5 years has been suggested for this group of patients with Kawasaki disease in the recently revised American Heart Association recommendations.41 Whether strategies to reduce arterial stiffness and ways to improve endothelial function would benefit patients with Kawasaki disease in the long term are topics for further research.
What is already known on the topic
Increased carotid intima‐media thickness has been reported in patients with Kawasaki disease and coronary artery lesions.
Central and peripheral arterial stiffening occurs in children after Kawasaki disease.
What this study adds
Carotid intima‐media thickness (IMT) is increased even in patients with Kawasaki disease without coronary sequelae.
A significant association is found between carotid IMT and systemic arterial stiffness in children after Kawasaki disease.
Abbreviations
HDL - high‐density lipoprotein
IMT - intima–media thickness
LDL - low‐density lipoprotein
PWV - pulse‐wave velocity
Footnotes
Competing interests: None declared.
References
- 1.Dhillon R, Clarkson P, Donald A E.et al Endothelial dysfunction late after Kawasaki disease. Circulation 1996942103–2106. [DOI] [PubMed] [Google Scholar]
- 2.Newburger J W, Burns J C, Beiser A S.et al Altered lipid profile after Kawasaki syndrome. Circulation 199184625–631. [DOI] [PubMed] [Google Scholar]
- 3.Silva A A, Maeno Y, Hashmi A.et al Cardiovascular risk factors after Kawasaki disease: a case‐control study. J Pediatr 2001138400–405. [DOI] [PubMed] [Google Scholar]
- 4.Cheung Y F, Yung T C, Tam S C F.et al Novel and traditional cardiovascular risk factors in children after Kawasaki disease. J Am Coll Cardiol 200443120–124. [DOI] [PubMed] [Google Scholar]
- 5.Noto N, Okada T, Yamasuge M.et al Noninvasive assessment of the early progression of atherosclerosis in adolescents with Kawasaki disease and coronary artery lesion. Pediatrics 20011071095–1099. [DOI] [PubMed] [Google Scholar]
- 6.Slyper A H. What vascular ultrasound testing has revealed about pediatric atherogenesis, and a potential clinical role for ultrasound in pediatric risk assessment. J Clin Endocrinol Metab 2004893089–3095. [DOI] [PubMed] [Google Scholar]
- 7.Greenland P, Abrams J, Aurigemma G P.et al Prevention conference V. Beyond secondary prevention: identifying the high‐risk patient for primary prevention, noninvasive tests of atherosclerotic burden: Writing Group III. Circulation 2000101E16–E22. [DOI] [PubMed] [Google Scholar]
- 8.Ooyanagi R, Fuse S, Tomita H.et al Pulse wave velocity and ankle brachial index in patients with Kawasaki disease. Pediatr Int 200446398–402. [DOI] [PubMed] [Google Scholar]
- 9.Senzaki H, Chen C H, Ishido H.et al Arterial haemodynamics in patients after Kawasaki disease. Circulation 20051112119–2125. [DOI] [PubMed] [Google Scholar]
- 10.Lakatta E C, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises. Part I: aging arteries: a “set up” for vascular disease, Circulation 2003107139–146. [DOI] [PubMed] [Google Scholar]
- 11.Wada T, Kodaira K, Fujishiro K.et al Correlation of ultrasound‐measured common carotid artery stiffness with pathological findings. Arterioscler Thromb 199414479–482. [DOI] [PubMed] [Google Scholar]
- 12.Athanassopoulos G, Olympios C, Foussas S.et al Atheromatous plaques in the thoracic aorta are associated with decreased aortic distensibility evaluated with transesophageal echocardiography and automatic boundaries detection. J Am Coll Cardiol 1994146A878–887. [Google Scholar]
- 13.Maarek B, Simon A C, Levenson J.et al Heterogeneity of the atherosclerotic process in systemic hypertension poorly controlled by drug treatment. Am J Cardiol 198759414–417. [DOI] [PubMed] [Google Scholar]
- 14.Herrington D M, Brown W V, Mosca L.et al Relationship between arterial stiffness and subclinical aortic atherosclerosis. Circulation 2004110432–437. [DOI] [PubMed] [Google Scholar]
- 15.Cheung Y F, Ho M H K, Tam S C F.et al Increased high sensitivity C reactive protein concentrations and increased arterial stiffness in children with a history of Kawasaki disease. Heart 2004901281–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirai T, Sasayama S, Kawasaki T.et al Stiffness of systemic arteries in patients with myocardial infarction: a noninvasive method to predict severity of coronary atherosclerosis. Circulation 19898078–86. [DOI] [PubMed] [Google Scholar]
- 17.Cheung Y F, Taylor M J O, Fisk N M.et al Fetal origins of reduced arterial distensibility in the donor twin in twin‐twin transfusion syndrome. Lancet 20003551157–1158. [DOI] [PubMed] [Google Scholar]
- 18.Nichols W W, O'Rourke M F. eds. McDonalds's blood flow in arteries: theoretical, experimental and clinical principles. London: Edward Arnold, 1998243–293.
- 19.Cerne A, Kranjec I. Atherosclerotic burden in coronary and peripheral arteries in patients with first clinical manifestation of coronary artery disease. Heart Vessels 200216217–226. [DOI] [PubMed] [Google Scholar]
- 20.Chambless L E, Heiss G, Folsom A R.et al Association of coronary heart disease incidence with carotid arterial wall thickness and major cardiovascular risk factors: the atherosclerosis risk in communities [ARIC] study, 1987–1993. Am J Epidemiol 1997146483–494. [DOI] [PubMed] [Google Scholar]
- 21.Tounian P, Aggoun Y, Dubern B.et al Presence of increased stiffness of the common carotid artery and endothelial dysfunction in severely obese children: a prospective study. Lancet 20013581400–1404. [DOI] [PubMed] [Google Scholar]
- 22.Pauciullo P, Iannuzzi A, Sartorio R.et al Increased intima‐media thickness of the common carotid artery in hypercholesterolemic children. Arterioscler Thromb 1994141075–1079. [DOI] [PubMed] [Google Scholar]
- 23.Jarvisalo M J, Putto‐Laurila A, Jartti L.et al Carotid artery intima‐media thickness in children with type 1 diabetes. Diabetes 200251493–498. [DOI] [PubMed] [Google Scholar]
- 24.Cuomo S, Guarini P, Gaeta G.et al Increased carotid intima‐media thickness in children‐adolescents, and young adults with a parental history of premature myocardial infarction. Eur Heart J 2002231345–1350. [DOI] [PubMed] [Google Scholar]
- 25.Ikemoto Y, Ogino H, Teraguchi M.et al Evaluation of preclinical atherosclerosis by flow‐mediated dilatation of the brachial artery and carotid artery analysis in patients with a history of Kawasaki disease. Pediatr Cardiol 200526782–786. [DOI] [PubMed] [Google Scholar]
- 26.Ishizu T, Ishimitsu T, Yanagi H.et al Effect of age on carotid arterial intima‐media thickness in childhood. Heart Vessels 200419189–195. [DOI] [PubMed] [Google Scholar]
- 27.Iemura M, Ishii M, Sugimura T.et al Long term consequences of regressed coronary aneurysms after Kawasaki disease: vascular wall morphology and function. Heart 200083307–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McConnell M E, Hannon D W, Steed R D.et al Fatal obliterative coronary vasculitis in Kawasaki disease. J Pediatr 1998133259–261. [DOI] [PubMed] [Google Scholar]
- 29.Amano S, Hazama F, Hamashima Y. Pathology of Kawasaki disease: I. Pathology and morphogenesis of the vascular changes. Jpn Circ J 197943633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farrar D J, Bond M G, Riley W A.et al Anatomic correlates of aortic pulse wave velocity and carotid artery elasticity during atherosclerosis progression and regression in monkeys. Circulation 1991831754–1763. [DOI] [PubMed] [Google Scholar]
- 31.van Popele N M, Grobbee D E, Bots M L.et al Association between arterial stiffness and atherosclerosis: the Rotterdam study. Stroke 200132454–460. [DOI] [PubMed] [Google Scholar]
- 32.Oren A, Vos L E, Uiterwaal C S P M.et al Aortic stiffness and carotid intima‐media thickness: two independent markers of subclinical vascular damage in young adults? Eur J Clin Invest 200333949–954. [DOI] [PubMed] [Google Scholar]
- 33.Megnien J L, Simon A, Denarie N.et al Aortic stiffening does not predict coronary and extracoronary atherosclerosis in asymptomatic men at risk for cardiovascular disease. Am J Hypertens 199811293–301. [DOI] [PubMed] [Google Scholar]
- 34.Zureik M, Temmar M, Adamopoulos C.et al Carotid plaques, but not common carotid intima‐media thickness, are independently associated with aortic stiffness. J Hypertens 20022085–93. [DOI] [PubMed] [Google Scholar]
- 35.Bots M L, Hofman A, Grobbee D E. Increased common carotid intima‐media thickness: adaptive response or a reflection of atherosclerosis? Findings from the Rotterdam Study. Stroke 1997282442–2447. [DOI] [PubMed] [Google Scholar]
- 36.Jogestrand T, Eiken O, Nowak J. Relation between the elastic properties and intima‐media thickness of the common carotid artery. Clin Physiol Func Imaging 200323134–137. [DOI] [PubMed] [Google Scholar]
- 37.Hong M K, Vossoughi J, Mintz G S.et al Altered compliance and residual strain precede angiographically detectable early atherosclerosis in low‐density lipoprotein receptor deficiency. Arterioscler Thromb Vasc Biol 1997172209–2217. [DOI] [PubMed] [Google Scholar]
- 38.Katsuda S, Hasegawa M, Kusanagi M.et al Comparison of pulse‐wave velocity in different aortic regions in relation to the extent and severity of atherosclerosis between young and older Kurosawa and Kusanagi‐hypercholesterolemic (KHC) rabbits. Clin Sci (Lond) 200099393–404. [PubMed] [Google Scholar]
- 39.Demer L L. Effect of calcification on in vivo mechanical response of rabbit arteries to balloon dilation. Circulation 1991832083–2093. [DOI] [PubMed] [Google Scholar]
- 40.Lyon R T, Runyon‐Hass A, Davis H R.et al Protection from atherosclerotic lesion formation by reduction of artery wall motion. J Vasc Surg 1987559–67. [PubMed] [Google Scholar]
- 41.Newburger J W, Takahashi M, Gerber M A.et al Diagnosis, treatment, and long‐term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Pediatrics 20041141708–1733. [DOI] [PubMed] [Google Scholar]