Figure 2. Elicitation of heat shock proteins (HSPs) by pustulan is due to β-1,6-glucan.
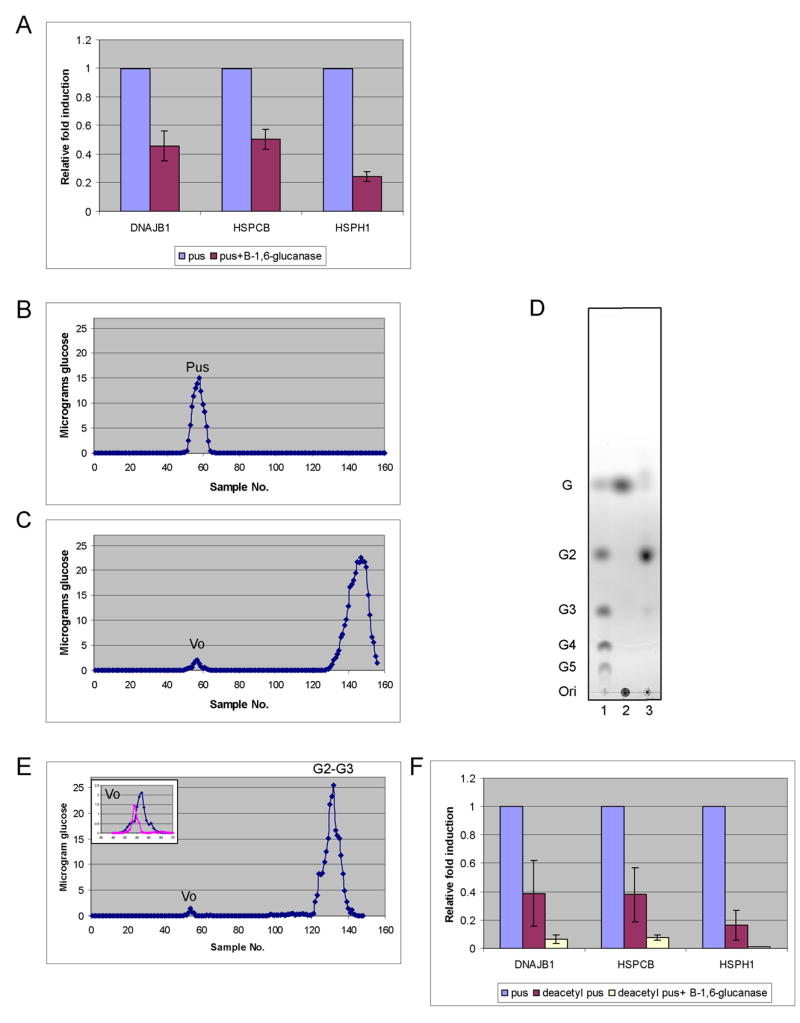
Neutrophils were cultured with opsonized beads for 2 hours at 37 °C in A and F. (A) Endo-β-1,6-glucanase reduces the induction of HSPs by pustulan. Beads were coated with an equivalent amount of pustulan (pus) or endo-β-1,6-glucanase digested pustulan. Induction of HSPs with enzyme treated pustulan is relative to that with untreated. The data represent the average of three experiments with standard deviation. (B) Pustulan chromatographed on Biogel P6 column. (C) Pustulan digested first with endo-β-1,6-glucanase and run on a P6 column generated a large and a small peak. The small peak represents a tiny fraction of the original pustulan that was resistant to enzymatic digestion (Vo). (D) The large peak in C was shown by thin layer chromatography to be the expected degradation products, gentiobiose and gentiotriose. Lane 1 contains standard oligosaccharides (G to G5) as controls. Lane 2 is pustulan spiked with glucose (G). Lane 3 is endo-β-1,6-glucanase digested pus. Ori = origin. (E) Chromatography of deacetylated pustulan. The insert is an overlay of the Vo from C and E. (F) Deacetylation of pustulan followed by digestion with endo-β-1,6-glucanase eliminates induction of HSPs. Induction of HSPs in deacetylated pustulan or deacetylated pustulan digested with endo-β-1,6-glucanase is relative to that with untreated pustulan.
