Fig. 3. The Roc domain is responsible for LRRK2 GTPase activity.
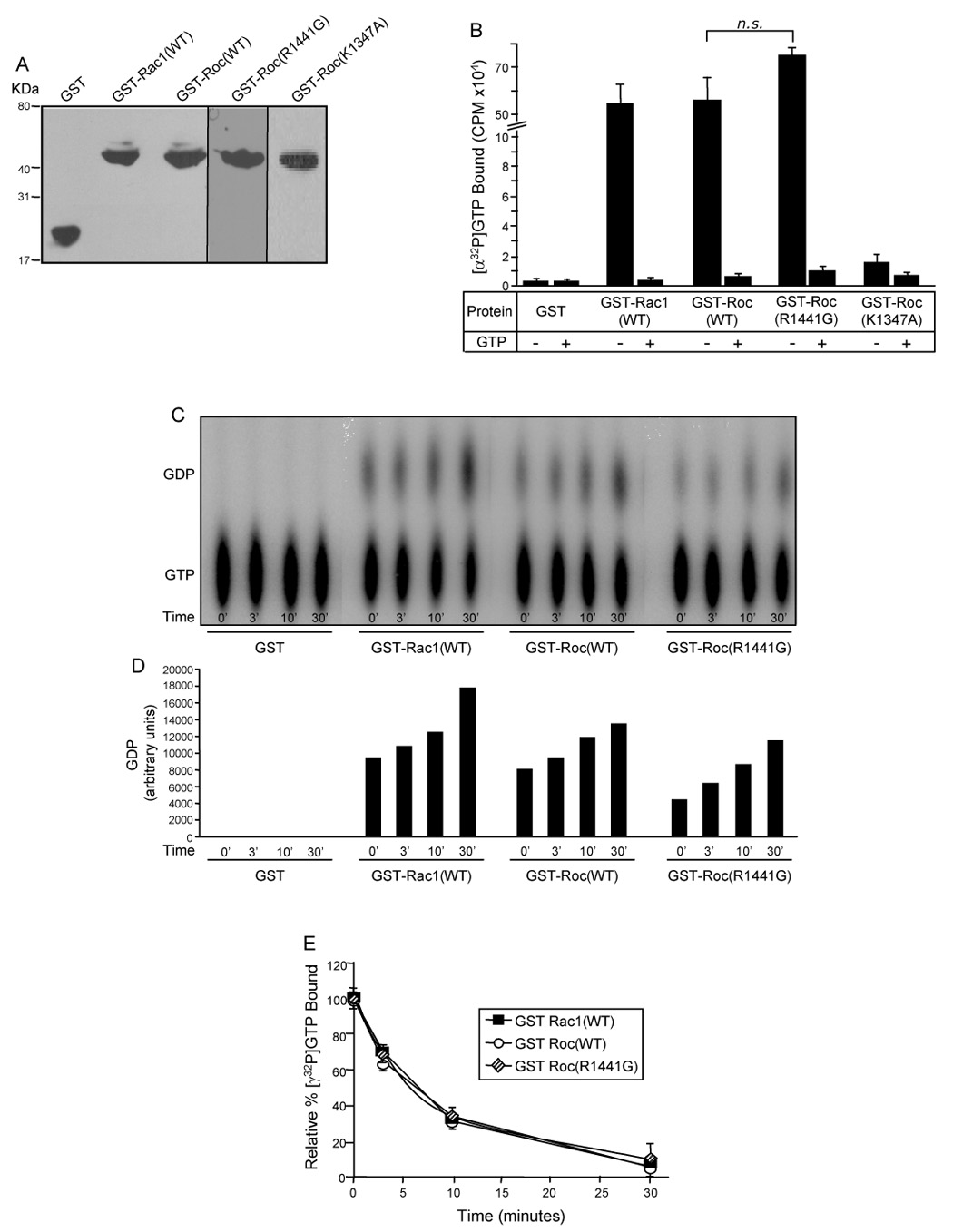
(A) Western blot of GST-fusion proteins. Purified GST fusion proteins were resolved by 12.5% Tris-glycine SDS-PAGE, transferred to PVDF membrane and Western blotted with anti-GST antibody to demonstrate equivalent amounts of protein utilized in the GTP-binding and hydrolysis assays. (B) GTP-binding to the GST-Roc domain of LRRK2. Purified recombinant GST-fusion proteins were assayed for [α-32P]GTP binding in the absence (−) or presence (+) of competing nucleotide (unlabeled GTP). CPM of [α-32P]GTP bound were plotted. (C) GTP hydrolysis of the GST-Roc domain of LRRK2 analyzed by the TLC method. GST-fusion proteins were assayed for [α-32P]GTP hydrolysis at 0, 3, 10 and 30mins by spotting on TLC plates. The autoradiograph shown is representative of three independent experiments. (D) Quantification of GTP hydrolysis of the GST-Roc domain of LRRK2 – TLC method. Amounts of GDP on the representative autoradiograph, shown in Fig. 3C, were quantified by densitometry. (E) GTP hydrolysis of the GST-Roc domain of LRRK2 – infinite dilution and rapid filtration method. GST-fusion proteins were incubated with [γ-32P]GTP and equivalent aliquots were removed at 0, 3, 10 and 30mins. The relative percent of [γ-32P]GTP bound, after subtracting background binding to GST alone (negative control), were plotted. CPM of [γ-32P]GTP bound at time zero was set to 100%. For all experiments GST and GST-Rac1(WT) were used as negative and positive controls, respectively. Error bars represent SEM for one experiment performed in triplicate. Similar results were observed in three independent experiments performed in triplicate. n.s. is non-significant compared to GST-Roc(WT), assessed by a two-tailed unpaired Student’s t-test.
