Fig. 4. LRRK2 kinase activity is stimulated by GTP-binding.
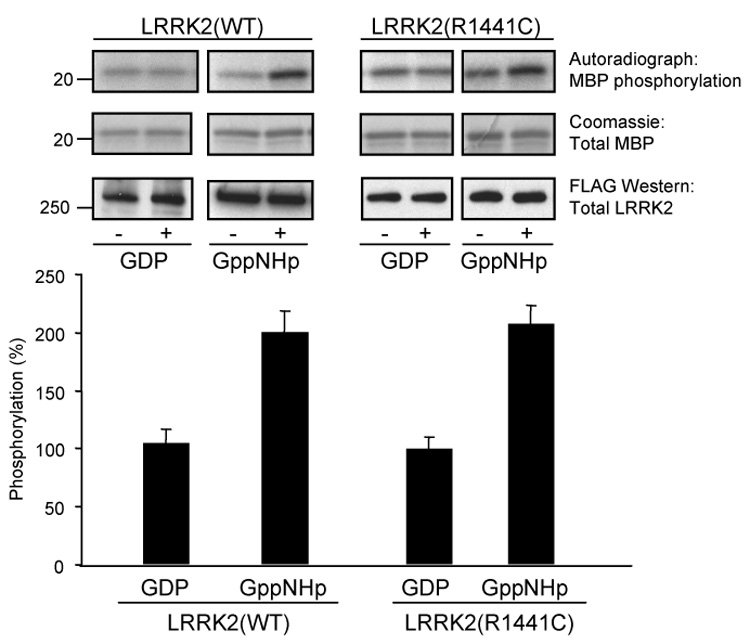
Immunoprecipitated LRRK2 was assayed for phosphorylation of MBP in the absence or presence of 5 µM of either GDP or a non-hydrolyzable GTP analog, Gpp(NH)p. Autoradiographs demonstrate incorporated 32P in MBP resolved by 12% Tris-glycine SDS-PAGE (top panel). MBP levels were determined by Coomassie staining (middle panel) and LRRK2 protein levels were determined by Western blot analysis using anti-FLAG antibody (bottom panel). Autoradiographs of 32P incorporated MBP (top panel) were quantified by densitometry and normalized to LRRK2 protein expression levels (bottom panel). LRRK2 trans-phosphorylation activity in the presence of GDP was set to 100%. Error bars represent SEM for three independent experiments.
