Figure 3.
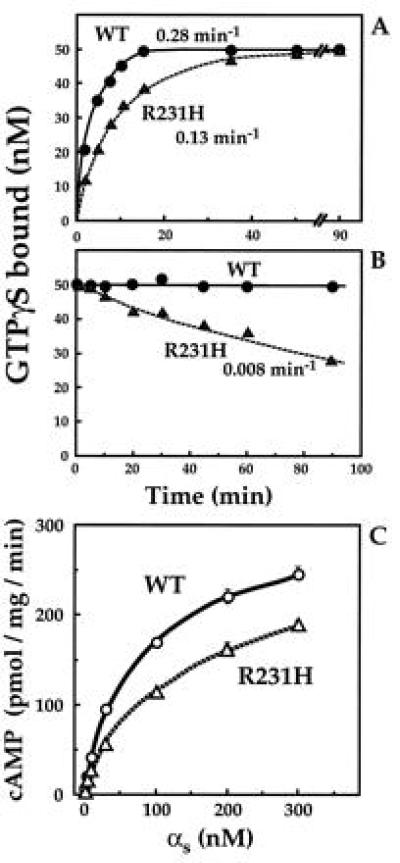
Biochemical properties of recombinant wt and mutant αs. (A) Rates of GTP[γS] binding. αs or αs-R231H (• and ▴, respectively; 50 nM each) were incubated at 22°C with 1 μM [35S]GTP[γS] (3 × 105 cpm/pmol). At the times indicated, the reaction was terminated and GTP[γS] binding was quantitated by filtration on nitrocellulose filters (13, 20) and apparent on-rates of GTP[γS] binding (kapp) were calculated (19). (B) Rates of dissociation of GTP[γS]. αs or αs-R231H (• and ▴, respectively; 50 nM each) were incubated at 22°C with 1 μM [35S]GTP[γS] as described in A for 45 min. Dissociation of bound [35S]GTP[γS] was assessed by adding 200 μM unlabeled GTP[γS] (at time zero in B). At the times indicated, the reaction was terminated and GTP[γS] binding was quantitated as described in A. (C) cAMP synthesis stimulated by different concentrations of αs or αs-R231H in the presence of GTP[γS]. Reactions were conducted at 22°C for 15 min in 50 μl volumes containing 15 μg cyc− membranes, as described (13). Before the assay, the αs proteins were incubated with 100 μM GTP[γS] for 60 min.
