Figure 4.
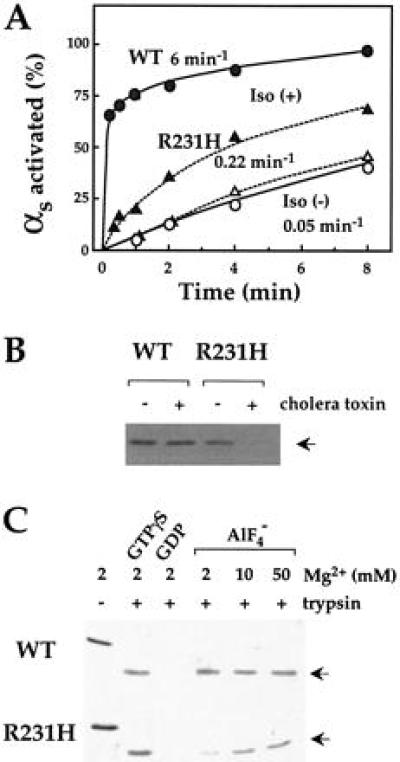
Effect of activation on tryptic cleavage of wt and mutant αs. (A) Receptor dependent activation of wt and mutant αs. Membranes (0.2 mg/ml) of COS-7 cells expressing recombinant HA-αs (•) or HA-αs-R231H (▴) plus the β2-AR and G protein β2 and γ2 subunits were incubated at 22°C with (filled symbols) or without (open symbols) 10 μM isoproterenol plus 10 μM GTP[γS]. At the times indicated, the reaction was terminated and samples were treated with trypsin (0.6 mg/ml) as described in Materials and Methods. Trypsin-resistant fragments of αs were visualized and quantitated by Western blot analysis using 12CA5 antibody (12). (B) Effect of modification by cholera toxin on protection by GTP[γS] against cleavage by trypsin. HEK293 cells stably transfected with HA-αs or HA-αs-R231H were cultured in the absence or in the presence of 1 μg/ml of cholera toxin for 3 h. Membranes were incubated with 10 μM GTP[γS] at 22°C for 10 min. Samples were incubated with trypsin (10 μg/ml) and trypsin-resistant fragments of αs (indicated by arrow) were visualized by Western blot analysis as described in A. (C) Effect of GTP[γS] and GDP/AlF4− on tryptic cleavage. αs or αs-R231H (0.7 μM each) were incubated with 10 μM GTP[γS], 10 μM GDP, or 10 μM GDP plus 20 μM AlCl3 and 10 mM NaF at 22°C for 60 min. Samples were further incubated in the absence or presence of trypsin (0.1 mg/ml) on ice for 60 min and trypsin-resistant fragments of αs (arrows) were visualized by SDS/PAGE followed by Coomassie blue staining.
