Figure 5.
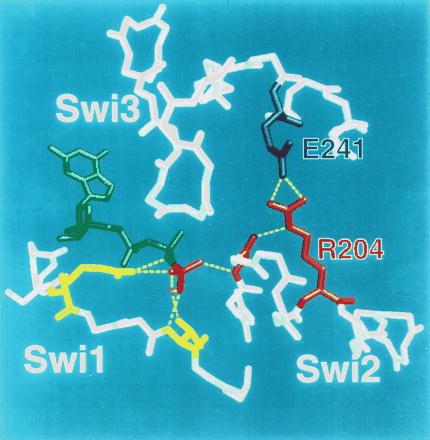
The arginine-glutamate hasp of Gα. Switch (Swi) regions 1–3 in the crystal structure of αt⋅GTP[γS] (3) are shown in relation to bound GTP (green; γ-phosphate colored red) and Mg2+ ion (magenta). Except for side chains of key amino acids, only main chains are depicted. The γ-phosphate of GTP (red) is linked to two amino acids (yellow, not labeled) in switch 1; these include R174 (on the left side of the picture) and T177. R174 corresponds to R201 of αs, the target of ADP ribosylation by cholera toxin. T177 (T 204 in αs) is linked to the γ-phosphate via bound Mg2+ (magenta). The γ-phosphate is linked to switch 2 by the main chain amide of G199 in αt (red, not labeled; corresponding to G226 in αs). The intramolecular hasp is the salt bridge between R204 (red; cognate to R231 in αs) in switch 2 (the α2 helix) and E241 (blue; E268 of αs) in α3. The hasp helps to maintain both the tight binding of GTP and the GTP-induced active conformation by fastening switch 2 and α3, thus reinforcing linkage of the glycine residue in switch 2 to the γ-phosphate of GTP.
