Figure 3. Inhibition of NFATc blocks TGFβ induction of fibronectin transcription.
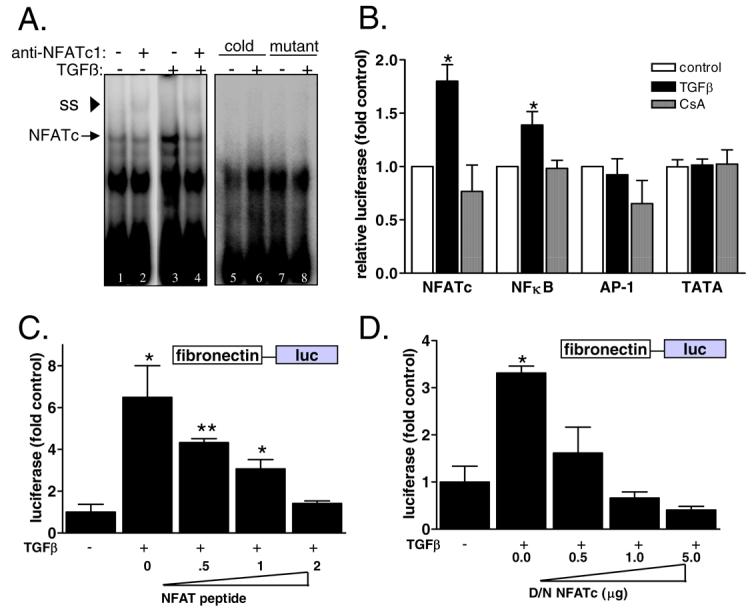
A) NFATc DNA binding in response to TGFβ was determined by electromobility shift assay. Mesangial cells were treated with SFM or TGFβ and then nuclear proteins were extracted and incubated with γ32P-labeled consensus NFATc probe or a mutant oligo. Finally, an antibody specific for NFATc1 was added. DNA/protein complexes were resolved by nondenaturing PAGE and results were visualized by autoradiography. Antibody binding was evident as a super-shifted (SS) band (asterisk). B) Mesangial cells were transiently transfected with an NFATc-responsive promoter sequence upstream of luciferase (NFATc-luc), AP1-luc, NFkB-luc, and TATA-luc along with a CMV-promoter driven renilla construct as a control for transfection. Cells were treated with SFM, TGFβ, or CsA+TGFβ and then activity of the target promoters were assessed by luciferase activity normalized by renilla. Data shown are the mean +/− SEM of triplicate samples. *p<0.05 C) Mesangial cells that stably express Fibro-luc were transiently transfected with increasing amounts of an NFATc inhibitory peptide and then fibronectin promoter activity in response to TGFβ was determined by luciferase activity normalized by protein concentration. Data shown are the mean +/− SEM of triplicate samples. * p<0.05 **p<0.01 D) Fibro-luc mesangial cells were transiently transfected with increasing amounts of a dominant negative NFATc cDNA and then fibronectin promoter activity in response to TGFβ was determined by luciferase activity normalized by protein concentration. Data shown are the mean +/− SEM of triplicate samples. * p<0.05.
