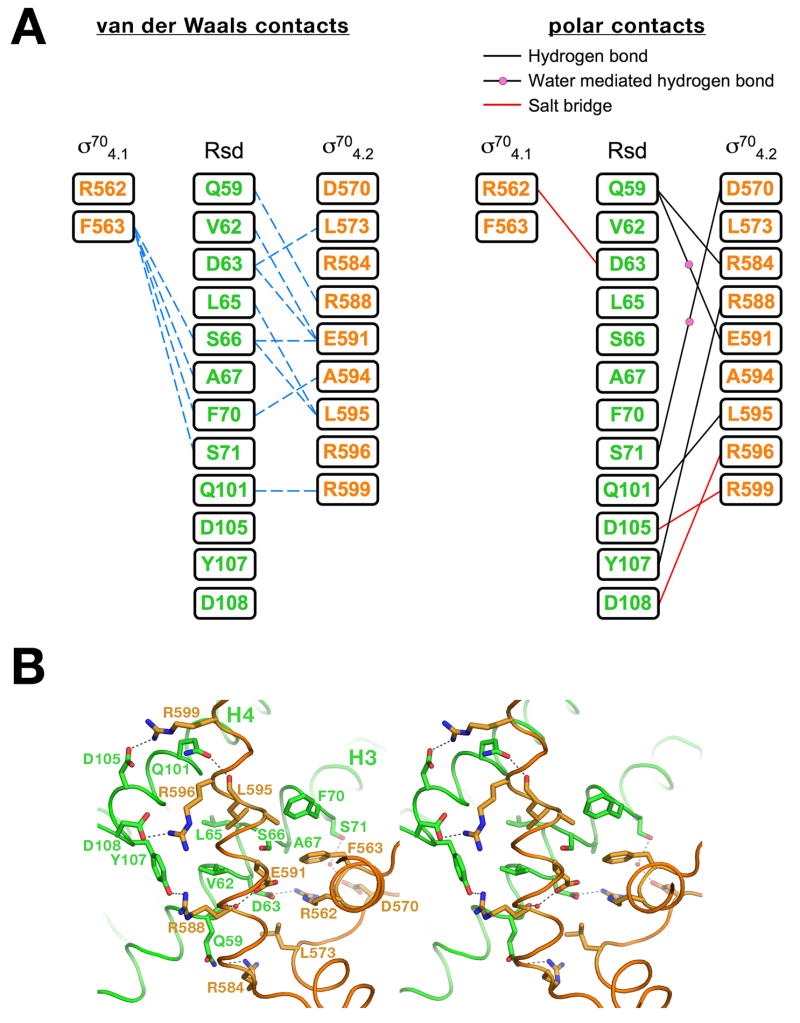
Figure 3. The σ704/Rsd interactions.
A) Schematic diagram denoting molecular interactions between Rsd and σ704. Van der Waals interactions (<4 Å) are listed on the left, polar interactions (hydrogen bonds, salt bridges) are listed on the right.
B) Stereo view of the σ704/Rsd interface. Protein α-carbon backbones are shown as worms, color-coded as in Figure 1. The two α-helices of Rsd harboring amino acid residues that interact with σ704 (H3 and H4) are labeled. Amino acid side chains that participate in interprotein interactions are shown, with carbon atoms color-coded as the backbone worm, nitrogen atoms colored blue, and oxygen atoms colored red. Interprotein polar interactions (hydrogen bonds or salt bridges) are indicated by dashed lines.