Table 1.
Effect of BA derivatives on the chymotrypsin-like activity of 20S proteasome.
 | |||
|---|---|---|---|
| Compounds | Structures | IC50# (μg/ml) | |
| R1 | R2 | ||
| DSB*1 (2) | 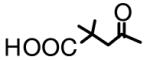 |
OH | 4.0 |
| A15*1 (3) |  |
OH | 8.2 |
| A16*1 (4) | OH | >20 | |
| A18*1 (5) | OH | >20 | |
| LH68*1 (6) | 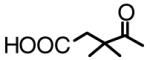 |
OH | 6.5 |
| LH141*2 (7) |
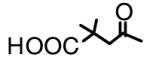 |
>20 | |
| IC9564*2 (8) |
H | >20 | |
