Abstract
Superior performance on the Embedded Figures Task (EFT) has been attributed to weak central coherence in perceptual processing in Autism Spectrum Disorders (ASD). The present study used functional magnetic resonance imaging to examine the neural basis of EFT performance in 7-12 year old ASD children and age and IQ matched controls. ASD children activated only a subset of the distributed network of regions activated in controls. In frontal cortex, control children activated left dorsolateral, medial and dorsal premotor regions whereas ASD children only activated the dorsal premotor region. In parietal and occipital cortices, activation was bilateral in control children but unilateral (left superior parietal and right occipital) in ASD children. Further, extensive bilateral ventral temporal activation was observed in control, but not ASD children. ASD children performed the EFT at the same level as controls but with reduced cortical involvement, suggesting that disembedded visual processing is accomplished parsimoniously by ASD relative to typically developing brains.
Despite deficits in multiple functional domains including social interaction, language, and executive functioning, individuals with Autism Spectrum Disorders (ASD) exhibit a notable strength, namely, superior ability to identify local features of complex visual stimuli (Happe & Frith, 2006). This perceptual superiority is most consistently reflected in faster and/or more accurate performance on the Embedded Figures Task (EFT), which requires identification of a simple shape embedded within a complex figure (Jolliffe & Baron-Cohen, 1997; Shah & Frith, 1983). An influential theoretical interpretation of this and similar findings on other visual-spatial tasks has been the proposal that individuals with ASD have a perceptual processing style that facilitates visual processing of local rather than global information, termed weak central coherence (Happe & Frith, 2006). Such an information processing style is consistent with symptoms of preoccupation with object parts and difficulty perceiving whole objects following changes in constituent parts (Happe & Frith, 2006). Regarding the EFT, the task draws upon the natural bias towards local visual processing in ASD, thereby yielding better performance in ASD than controls. In contrast, typically developing individuals are slower and/or less accurate on the EFT because disembedded visual processing conflicts with their natural bias. Behavioral studies indicate that typically developing individuals perceive or attend to the global form of a figure before its local components (Kimchi, 1992). Overriding that global perceptual bias to disembed a local component during the EFT is likely to be more effortful for control than ASD individuals (Happe & Frith, 2006). Attentional bias toward local features in ASD, but global form in typical development, suggests that visual perception differs qualitatively between these groups.
Two main findings have emerged about the functional anatomy of local/global perceptual processing from studies using hierarchical stimuli (e.g., an “H” composed of small “E”s) and the EFT. Hierarchical stimuli allow differential examination of local/global processing by directing attention to either global (e.g., detect “H”) or local (e.g., detect “E”) elements of the stimuli. In contrast, the EFT directs attention to local elements of a visually complex figure and although global form is likely to be perceived, its detection is not required for task performance. First, studies of hierarchical stimuli with visual-field behavioral methods in healthy (Blanca et al., 1994) and brain-damaged (Robertson & Lamb, 1991) adults and functional brain imaging in healthy adults (Fink et al., 1996, 1997; Lux et al., 2004) indicated that posterior cortical recruitment (temporoparietal and occipital) was lateralized to the right hemisphere during global processing and to the left hemisphere during local processing. Functional brain imaging of healthy adults and adolescents performing the EFT revealed recruitment of occipito-parietal cortex bilaterally (Manjaly et al., 2007; Manjaly et al., 2003; Ring et al., 1999), but was restricted to left parietal cortex when the EFT was contrasted with a control condition in which subjects viewed the complex form with the local component highlighted (Manjaly et al., 2007; Manjaly et al., 2003). Thus, left parietal involvement appears to be specific to the disembedded visual processing required for the EFT. Taken together, these studies suggest that posterior cortical involvement in the left hemisphere relates to perception of local elements of a visual form.
Second, frontal cortical involvement was medial during local relative to global processing of hierarchical stimuli (Lux et al., 2004; Weissman et al., 2003; Weissman et al., 2005) and in right dorsolateral prefrontal cortex during the EFT (Ring et al., 1999). However, upon using a control condition that allowed selective visualization of disembedded processing on the EFT, activation was limited to left ventral premotor cortex (Manjaly et al., 2007; Manjaly et al., 2003). Medial frontal involvement during local processing has been posited to reflect the suppression of the bias toward global perceptual processing, based upon that region's role in conflict resolution in other studies (Lux et al., 2004). Dorsolateral frontal involvement during the EFT has been posited to reflect working memory demands relating to complex visual-spatial processing, whereas left premotor involvement may relate to response planning associated with disembedded visual processing. Thus, a number of frontal cortical regions contribute to perceptual processing on the EFT.
If perceptual biases differ between ASD and typically developing individuals, then the functional anatomy of EFT performance ought to differ between the two groups. Indeed, brain imaging studies comparing ASD and controls during EFT performance showed differences in adults (Ring et al., 1999) and adolescents (Manjaly et al., 2007). Relative to controls, ASD groups showed greater activation in posterior visual regions, in the right occipital cortex extending into inferior temporal gyrus in Ring et al. (1999) and in bilateral occipital cortices in Manjaly et al. (2007). In contrast, the control groups showed greater recruitment of other anterior and posterior regions, namely, right dorsolateral prefrontal and bilateral parietal cortices in Ring et al. (1999) and premotor and parietal cortices in the left hemisphere in Manjaly et al. (2007). Different findings between studies most likely reflect differences in experimental task design rather than age-related change between adolescence and adulthood because using the same experimental task, typically developing adolescents (Manjaly et al., 2007) and adults (Manjaly et al., 2003) showed similar recruitment of premotor and parietal cortices in the left hemisphere. The Manjaly et al. studies included control tasks that allowed selective imaging of disembedded visual processing (i.e., EFT vs. the local component outlined within the global form) whereas Ring et al. used a control task that allowed for imaging of a wider range of perceptual and cognitive processes (i.e., EFT vs. visual fixation to a blank screen). Nevertheless, despite variability in findings of posterior cortical differences between ASD and control groups, both studies converge in revealing reduced frontal recruitment in ASD relative to control subjects.
The present study used functional magnetic resonance imaging (fMRI) during EFT performance in pre-adolescent ASD and age and IQ matched typically developing children. Pre-adolescence was defined according to normative studies indicating that pubertal changes are incomplete until approximately 12-14 years of age (Patton & Viner, 2007). Findings from past EFT imaging studies in adults and adolescents may not extend to pre-adolescent children for two reasons: First, since lateralization of local/global processing is not complete until early adolescence, the pattern of hemispheric involvement is likely to be unique prior to adolescence. Although a global perceptual bias is present in infancy (Colombo et al., 1991), laterality differences in perceptual performance (Mondloch et al., 2003) and cortical recruitment (Moses et al., 2002) are not stable until early adolescence. Adult-like temporo-parietal lateralization during hierarchical processing was observed only in adolescents exhibiting an adult-like visual-field advantage (Moses et al., 2002). Thus, hemispheric specialization of posterior cortices characterizing adults and some adolescents ought to differ from that in pre-adolescent children. Second, frontal activation observed during local perceptual processing is thought to reflect executive operations such as visual-spatial working memory (Ring et al., 1999) and inhibition of global processing (Lux et al., 2004). Those operations are mediated by prefrontal cortex, which is structurally immature in childhood (Giedd, 2004) and differs functionally during visual-spatial working memory (Klingberg et al., 2002) and inhibitory (Bunge et al., 2002) tasks in pre-adolescent children relative to adults. Thus, frontal lobe involvement during local processing in pre-adolescent children may differ from that in adults and adolescents.
Our experimental design included some unique features relative to past EFT imaging studies. First, trial length and the number of trials per block depended upon subjects' performance (i.e., self-paced) rather than being fixed. Such a design ensured that functional images reflected the engagement of processes related to solving the EFT rather than to incomplete resolution or idle time following EFT completion. A self-paced design allows better detection of task-related neural processes in the event of individual variability in processing or response times (D'Esposito et al., 1997) which is greater in normative and disordered development (Berl et al., 2006). Second, our control task, matching of simple geometric shapes, controlled for motor and visual processes associated with shape comparison. Thus, activation during the EFT reflected brain functions related to visual perception of complex spatial information and disembedded processing.
Method
Subjects
Seventeen (12 males) ASD children aged 7-12 years (M = 10.37, SD = 1.52) with average IQ (M = 109.31, SD = 14.19) were recruited from a community-based outpatient clinic. Fourteen (11 males) control children aged 7-12 years (M = 10.85, SD = 1.47) with high average IQ (M = 115.08, SD = 11.62) were recruited through advertisements. Along with parental consent, all subjects gave assent and were paid $30 for their participation. The two groups did not differ in age (p = .43) or IQ (p = .27). ASD was diagnosed according to DSM-IV ((APA), 2000) criteria; eight of the children were diagnosed with high-functioning autistic disorder and nine of the children with Asperger's disorder. Diagnoses were confirmed with the Autism Diagnostic Interview – Revised (ADI-R) (Lord et al., 1994) in 11 children and the Autism Diagnostic Observation Schedule – Generic (ADOS-G) (Lord et al., 2000) in 10 children1. Exclusion criteria included: (a) Full Scale IQ below 85 as measured by the Wechsler Intelligence Scale for Children – Third Edition (WISC-III) or Wechsler Abbreviated Scale of Intelligence (WASI); (b) Reading disability as measured by Woodcock-Johnson – Third Edition (WJ-III) Letter Word Identification or Word Attack subtest standard scores below 85; and (c) Evidence of other neurological (e.g., epilepsy) or psychiatric (e.g., mood/anxiety) disorders. ASD children withheld stimulant medications for 36 hours prior to participation (n = 4), but not other psychotropics (atomoxetine = 1, citalopram = 1, clonidine = 3, quetiapine = 1, sertraline hydrochloride = 1); the remaining children were unmedicated. All the medications listed above are commonly used to treat symptoms of ASD and do not indicate the presence of comorbid psychiatric conditions.
Stimulus Materials
Stimuli were generated in Photoshop (Adobe Systems Incorporated, San Jose, CA). For the EFT, stimuli comprised 40 pairs of complex figures (12 from the original EFT (Witkin et al., 1971) and 68 new figures matched for visual complexity) that served as probe figures and 40 target shapes that were embedded within one of the probe figures. For the Matching Task (MATCH), stimuli comprised 50 pairs of simple figures (the 40 target shapes from the EFT and 60 additional shapes matched for visual complexity) that served as probe and target figures; although the 40 target shapes were shared between the EFT and the MATCH, target shapes were not used as distracters for the MATCH stimuli. In light of the self-paced design (described below), subjects were likely to complete more trials of the simpler MATCH than the more complex EFT. Therefore, more MATCH than EFT stimuli were required to ensure sufficient stimuli for trials. Each trial comprised one stimulus display (14 × 11 inches) consisting of a single simple target shape and two probe figures presented above it; the target shape was embedded within one of the probe figures for the EFT, but was identical to one of the probe figures for the MATCH (Figure 1). The letters “L” and “R” were presented below the probe figures as an indicator of the hand with which to respond for each probe figure. These labels were presented with each stimulus display to ensure that children did not forget their response choices and response mode.
Figure 1.
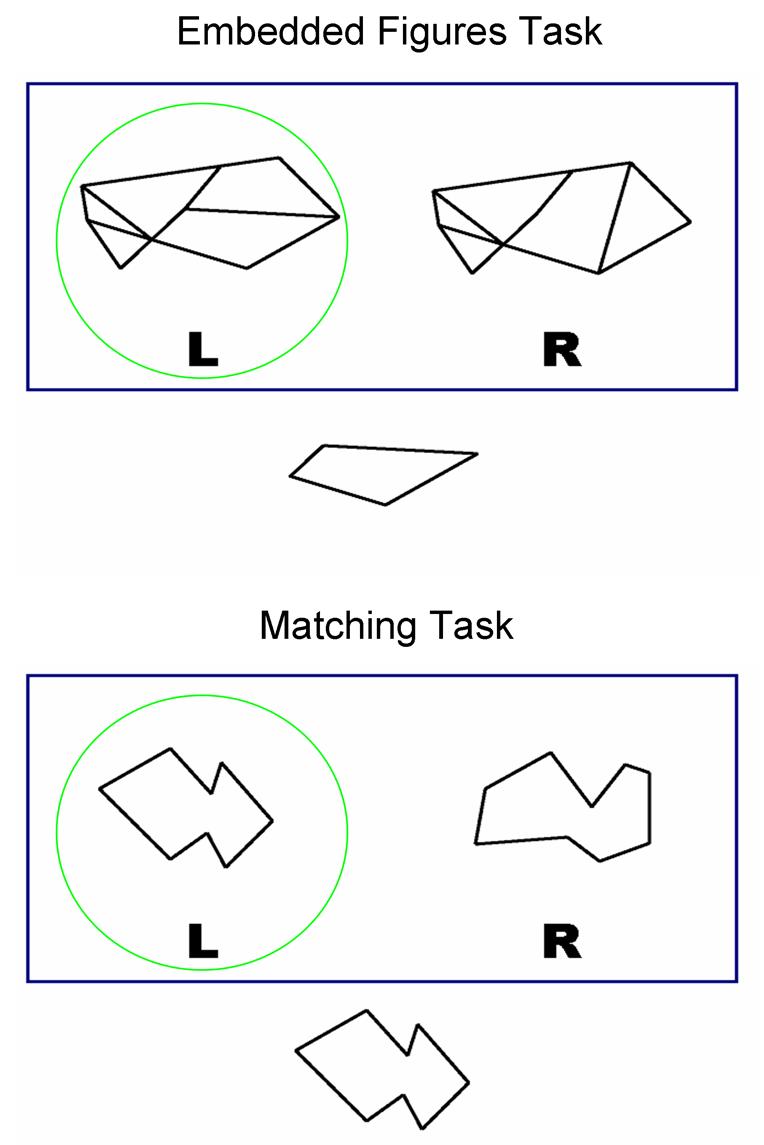
Examples of the stimulus display for the Embedded Figures (top) and Matching (bottom) Tasks. Correct answers circled for illustrative purposes.
Task Design
Stimuli were presented in ePrime (Psychological Software Tools, Inc., Pittsburgh, PA) and viewed through a magnet compatible projector. Responses were recorded via two fiber optic button boxes, one held in each hand. Head movement was minimized by small foam cushions placed on the sides of the subject's head.
Each subject performed one run lasting for five minutes. Trials were self-paced in alternating EFT and MATCH blocks. Within each block, stimulus displays were selected randomly by ePrime for each trial without repetition during the run; however, some target shapes occurred in both EFT and MATCH blocks. Subjects were instructed to examine the target shape and select the left or right probe figure that contained the target shape in the EFT blocks or was identical to the target shape in the MATCH blocks. Subjects indicated their response by pressing the button in the hand that corresponded to the left/right probe figure; the response also initiated the subsequent trial. Full counterbalancing of left and right button responses was not possible, since the stimuli were drawn at random by ePrime from the full set that had equal left and right responses for the EFT and MATCH. The percentage of left and right responses for the EFT (Left Hand – ASD: M = 50.10, SD = 5.09; Controls: M = 51.20, SD = 3.68; Right Hand – ASD: M = 49.90, SD = 5.09; Controls: M = 48.80, SD = 3.68) and the MATCH (Left Hand – ASD: M = 52.71, SD = 4.58; Controls: M = 50.21, SD = 4.32; Right Hand – ASD: M = 48.39, SD = 4.58; Controls: M = 49.79, SD = 4.32) did not differ between the groups (main effect of Group, ps > .41) and did not differ between the groups for responses with either hand (Group × Hand interaction, ps > .52). Subjects proceeded through the trials in each block at their own pace, and thus, the length and total number of trials within each block differed across subjects. However, the two types of blocks alternated such that each block lasted for at least 30 seconds. In the event that a subject had not yet responded to the trial that began prior to and extended past 30 seconds from the beginning of the block, the current trial continued until a response was given, allowing for blocks longer than 30 seconds. For instance, if a subject began a trial 29 seconds from the beginning of a block and responded 5 seconds later, that trial would have continued and the block would have lasted 34 seconds before switching to the first trial of the next block. The task terminated at five minutes, regardless of the number of trials or blocks completed. While it was plausible that a single trial/block would last the entire five minutes if no response was given, this did not occur for any subject. All subjects completed at least three blocks each of the EFT and the MATCH. The groups did not differ in number of blocks completed for the EFT (ASD: M = 3.53, SD = 0.28; Controls: M = 3.62, SD = 0.51) or the MATCH (ASD: M = 3.92, SD = 0.28; Controls: M = 3.85, SD = 0.38) (ps > .56). While such an experimental design is unusual, we reasoned that it was optimal for ensuring that visualized processes reflected EFT engagement and resolution without processing time limitations.
Imaging Procedure
A high-resolution sagittal T1-weighted structural scan was acquired on a Siemens Trio 3T MRI scanner (Erlangen, Germany) using a 3D MPRAGE sequence with a scan time of 6:51min and the following parameters: TR = 1600ms, TE = 4.4ms, 256×256mm FOV, 160mm slab with 1mm thick slices, 256×256×160 matrix (effective resolution of 1.0mm3), 1 excitation, and a 15 degree flip angle. Functional images were acquired on the same equipment using a T2*-sensitive gradient echo pulse sequence with the following parameters: TR = 2500ms, TE = 30ms, 256×256mm FOV, 64×64 acquisition matrix, and a 90 degree flip angle. Forty-two 3.7mm thick interleaved slices were acquired descending in the transverse plane (width = 3.7mm, gap width = 0.3mm, effective width = 4mm) for 98 time points (the first 2 TRs were included for signal stabilization and were discarded from analysis).
Data Analysis
Data were analyzed using SPM2 (Wellcome Department of Cognitive Neurology, London, UK) implemented in MATLAB (Version 7.0, Mathworks, Inc., Sherborn, MA). Subjects with greater than 3mm of translational motion in the x, y, or z directions throughout the course of their scan were not included in the analyses (four ASD and one control); therefore, the final imaging analysis consisted of 13 ASD and 13 control children (see Table 1 for sample characteristics). The lack of group differences in age and IQ remained in the final sample (ps > .33). Separate two sample t-tests indicated that the maximum translational displacement did not differ between the groups in any direction (X Translation – ASD: M = .39mm, SD = .44mm; Controls: M = .42mm, SD = .68mm; Y Translation – ASD: M = .59mm, SD = .36mm; Controls: M = .48mm, SD = .36mm; Z Translation – ASD: M = 1.00mm, SD = .76mm; Controls: M = 1.21; SD = 1.41mm) (ps > .17).
Table 1.
Individual subject characteristics and activations during the Embedded Figures Task relative to the Matching Task for Regions of Interest
| Subject Characteristic |
Region of Interest (Brodmann's Area) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Age | Gender | FSIQ | Diagnosis | 9/46 | 8/32 | 35/36/37 | 7 | 18 | |
| Control 1 | 12 | M | 129 | N.A. | Left | Left | Bilat. | Bilat. | Bilat. |
| Control 2 | 10 | M | 129 | N.A. | Left | Left | Bilat. | Bilat. | Bilat. |
| Control 3 | 11 | M | 114 | N.A. | Left | Left | Bilat. | Bilat. | Bilat. |
| Control 4 | 10 | M | 89 | N.A. | Left | Left | Bilat. | Bilat. | Bilat. |
| Control 5 | 11 | M | 115 | N.A. | Left | Left | Bilat. | Bilat. | Bilat. |
| Control 6 | 8 | M | 123 | N.A. | Left | Left | Bilat. | Bilat. | Bilat. |
| Control 7 | 9 | F | 121 | N.A. | Left | Left | Bilat. | Bilat. | Bilat. |
| Control 8 | 12 | M | 115 | N.A. | Left | Left | Bilat. | Left | Bilat. |
| Control 9 | 11 | M | 124 | N.A. | Left | Left | Bilat. | Left | Bilat. |
| Control 10 | 12 | M | 112 | N.A. | Left | Left | Bilat. | N.S. | Bilat. |
| Control 11 | 11 | F | 114 | N.A. | Left | Left | Bilat. | Left | Left |
| Control 12 | 12 | M | 96 | N.A. | Left | N.S. | Bilat. | Bilat. | Bilat. |
| Control 13 | 9 | M | 115 | N.A. | Left | N.S. | Bilat. | N.S. | Bilat. |
| ASD 1 | 11 | M | 119 | HFA | Left | Left | Bilat. | Bilat. | Bilat. |
| ASD 2 | 10 | M | 117 | ASP | Left | Left | Bilat. | Left | Bilat. |
| ASD 3 | 12 | F | 99 | HFA | Left | Left | Right | Bilat. | Bilat. |
| ASD 4 | 10 | F | 127 | HFA | Left | Left | N.S. | Left | Bilat. |
| ASD 5 | 8 | M | 119 | HFA | Left | N.S. | N.S. | Bilat. | Bilat. |
| ASD 6 | 7 | M | 109 | HFA | Left | N.S. | N.S. | Left | Right |
| ASD 7 | 8 | M | 99 | ASP | N.S. | N.S. | Bilat. | Left | Right |
| ASD 8 | 11 | M | 103 | ASP | N.S. | N.S. | Bilat. | N.S. | Right |
| ASD 9 | 9 | M | 126 | ASP | N.S. | N.S. | Right | Left | N.S. |
| ASD 10 | 11 | M | 121 | HFA | N.S. | N.S. | N.S. | Bilat. | Bilat. |
| ASD 11 | 11 | F | 112 | ASP | N.S. | N.S. | N.S. | N.S. | Bilat. |
| ASD 12 | 11 | M | 85 | HFA | N.S. | N.S. | N.S. | Left | Right |
| ASD 13 | 12 | M | 85 | ASP | N.S. | N.S. | N.S. | N.S. | Right |
N.A. = not applicable; HFA = High-Functioning Autistic Disorder; ASP = Asperger's Disorder; Bilat. = Bilateral; N.S. = not significant
For each subject, 10 temporally contiguous volumes were selected from each block for analysis, resulting in a total of 60 volumes, 30 for the EFT and 30 for the MATCH. In the event that more than 10 volumes were collected for a block due to the self-paced design, only the first 10 volumes were analyzed. Images were normalized into the MNI standard anatomical space and interpolated to 2×2×2mm cubic voxels. Normalized image volumes were spatially smoothed using an 8mm full width at half-maximum Gaussian kernel and temporally filtered (high-pass filter: SPM default calculated based upon trial frequency). fMRI responses were modeled by a canonical hemodynamic response function. For each subject, activation maps were generated using a linear contrast identifying regions that showed greater activation during EFT relative to MATCH blocks. Individual activation maps were averaged across subjects separately for the ASD and control groups in a random effects model (height threshold of p < .005 and cluster-level threshold of p < .05, uncorrected for multiple comparisons). Group differences in activation were assessed based on parameter estimates extracted from functionally defined regions of interest (ROI) using the MarsBar toolbox for SPM2 (Brett et al., 2003). This ROI analysis was limited to significantly activated regions that overlapped in spatial location on the group-averages of the two groups. One ROI was functionally defined for each overlapping cluster, by computing the conjunction of spatially overlapping clusters such that the ROI encompassed activated clusters from both groups. Parameter estimates were extracted by applying that conjuction-defined ROI to each individual subject and analyzed separately for each region using two-sample t-tests.
Results
Behavioral Results
For each subject, percentage of correct responses (accuracy), number of completed trials, and median response times were computed for the EFT and the MATCH blocks, including all completed trials and not only those included in the fMRI analysis. Separate repeated measures analyses of variance (ANOVAs) were computed for accuracy, total items completed, and response times with Group (ASD, Controls) as the between-subjects factor and Task (EFT, MATCH) as the within-subjects factor. Accuracy did not differ between the groups (EFT - ASD: M = 61.83, SD = 14.75; Controls: M = 65.02, SD = 11.66; MATCH - ASD: M = 88.07, SD = 7.05; Controls: M = 90.00, SD = 6.34) (main effect of Group, p = .44). Accuracy was lower on the EFT than on the MATCH (main effect of Task), F (1, 24) = 104.02, p < .001, but did not differ between the groups for either task (Group × Task interaction, p = .80). The number of completed trials did not differ between the groups (EFT - ASD: M = 29.69, SD = 26.70; Controls: M = 19.92, SD = 10.05; MATCH - ASD: M = 57.00, SD = 15.95; Controls: M = 57.08, SD = 5.06) (main effect of Group, p = .38). The number of completed trials was lower in the EFT than the MATCH blocks (main effect of Task), F (1, 24) = 83.72, p < .001, but did not differ between the groups for either task (Group × Task interaction, p = .18). Mean response times did not differ between the groups (EFT - ASD: M = 7.41s, SD = 6.52s; Controls: M = 5.80s, SD = 3.43s; MATCH - ASD: M = 1.62s, SD = 3.45s; Controls: M = 1.47s, SD = 1.67s) (main effect of Group, p = .41), were longer for the EFT than the MATCH (main effect of Task), F (1, 24) = 25.22, p < .001, but did not differ between the groups for either task (Group × Task interaction, p = .47). There appeared to be greater variability in the number of EFT trials completed for the ASD group. The relationship between response time and number of completed EFT trials is depicted in Figure 2. Overall, both groups performed less accurately, completed fewer trials, and required more time per trial for the EFT than the MATCH. Most importantly, however, ASD children did not differ from control children on any performance index on either the EFT or the MATCH.
Figure 2.
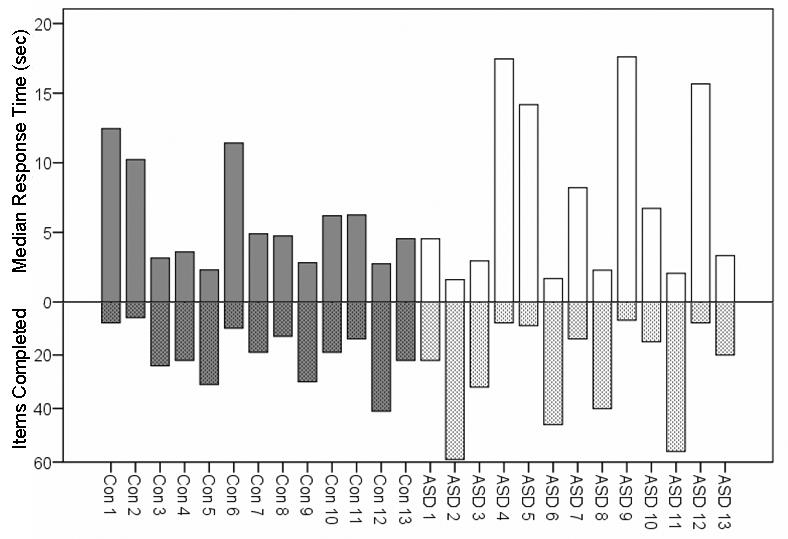
Individual subject median response time (solid bars) and number of items completed (cross-hatch bars) for the Embedded Figures Task.
Imaging Results
Significant areas of activation are listed in Table 2 and depicted in Figures 3 and 4. During EFT relative to MATCH performance, control children recruited frontal cortex exclusively in the left hemisphere, including inferior and middle frontal (BA 9/46 extending into BA 47 ventrally and BA 10 anteriorly) gyri and dorsal premotor cortex (BA 6) laterally, and anterior cingulate (BA 8/32) medially. In addition, control children activated multiple posterior regions including dorsal regions in bilateral superior parietal lobule (BA 7) and left inferior parietal lobule (BA 40), ventral regions in bilateral fusiform gyrus extending into left lingual gyrus (BA 37) and right parahippocampal gyrus (BA 35/36), and bilateral occipital cortex (BA 18). ASD children differed from controls in two respects: First, they failed to recruit medial and lateral prefrontal cortex, ventral temporal cortex, and inferior parietal cortex. Activation in these regions was not simply weak because it was also not observed at a lower threshold of p < .05. Second, in contrast to the bilateral parietal and occipital activations in control children, ASD children activated these regions unilaterally, specifically the superior parietal lobule (BA 7) in the left hemisphere and the occipital lobe (BA 18) in the right hemisphere. Furthermore, ROI analyses of those regions indicated a weak trend towards reduced activation in ASD relative to control children, left BA 7, t (24) = −1.67, p = .11, and right BA 18, t (24) = −1.86, p = .08. Pervasive reduction in activation, however, does not appear to characterize ASD children because they recruited left dorsal premotor cortex (BA 6) as much as control children (p = .22). Thus, EFT performance in ASD children was characterized by lack of prefrontal and ventral-temporal cortical recruitment and reliance on a subset of the parieto-occipital network activated in control children.
Table 2.
Regional activation during the Embedded Figures Task relative to the Matching Task in ASD and Control children
| Region of Activation | Brodmann's Area | Talairach Coordinates |
Volume (mm3) |
Z Score | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Control Group | ||||||
| Left superior frontal gyrus/anterior cingulate | 8/32 | −2 | 29 | 41 | 804 | 3.63 |
| Left premotor cortex | 6 | −26 | 11 | 55 | 149 | 3.40 |
| Left middle frontal gyrus | 10 | −34 | 57 | 6 | 139 | 4.26 |
| Left inferior frontal gyrus | 9/46 | −50 | 38 | −7 | 1182 | 3.81 |
| Left lingual/fusiform gyrus | 37 | −28 | −43 | −6 | 419 | 4.08 |
| Right parahippocampal/fusiform gyrus | 35/36 | 30 | −41 | −6 | 581 | 5.29 |
| Left superior parietal lobe | 7 | −22 | −60 | 44 | 510 | 3.97 |
| Right superior parietal lobe | 7 | 22 | −58 | 43 | 185 | 2.96 |
| Left inferior parietal lobe | 40 | −42 | −44 | 46 | 153 | 3.27 |
| Left occipital lobe | 18 | −22 | −93 | −2 | 969 | 5.38 |
| Right occipital lobe | 18 | 22 | −99 | 5 | 799 | 4.44 |
| ASD Group | ||||||
| Left premotor cortex | 6 | −20 | 14 | 51 | 474 | 4.29 |
| Left superior parietal lobe | 7 | −14 | −67 | 51 | 259 | 3.97 |
| Right occipital lobe | 18 | 24 | −97 | 1 | 131 | 3.88 |
Figure 3.
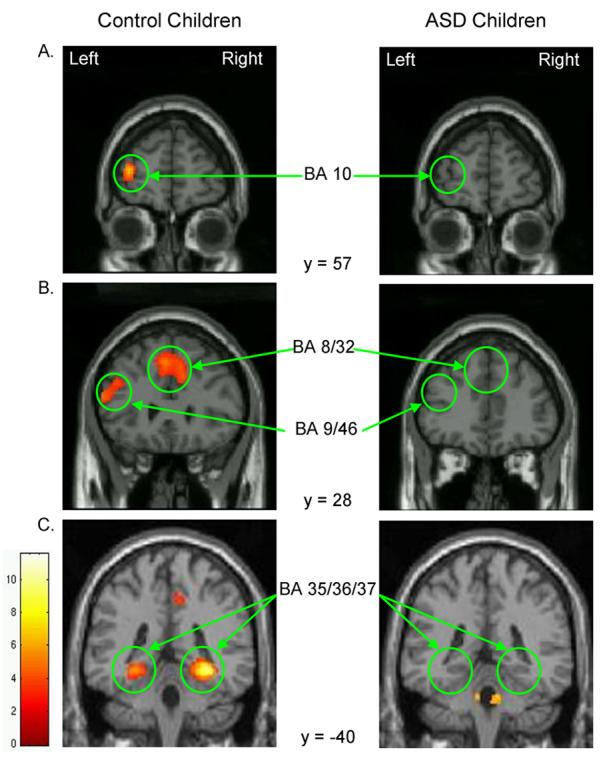
Coronal slices highlighting prefrontal (A, B) and ventral temporal (C) regions of activation that differed between ASD and control children.
Figure 4.
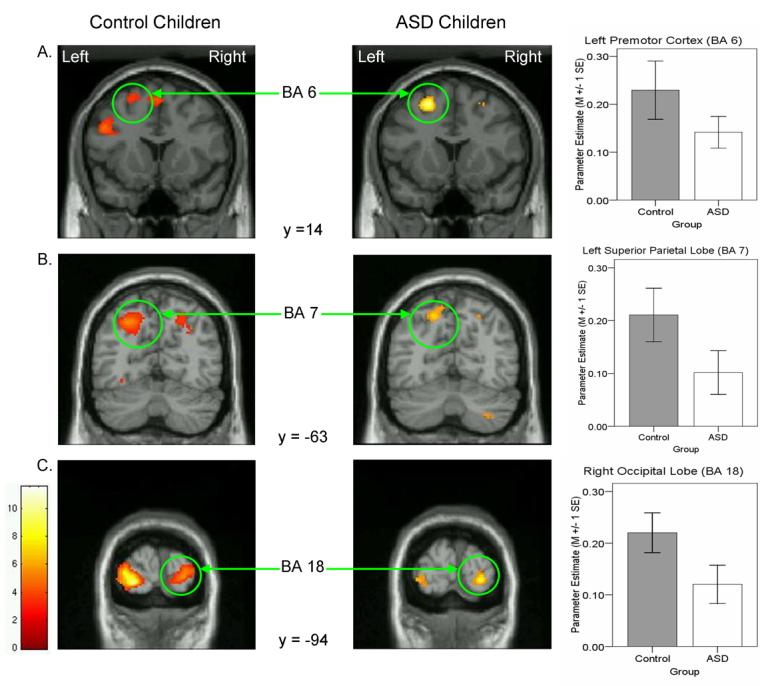
Coronal slices highlighting premotor (A), parietal (B), and occipital (C) regions of activation that were similar between ASD and control children. Adjoining graphs depict mean parameter estimate from the highlighted region of interest in ASD (open bars) and control (filled bars) children.
One reason for the absence or reduction of regional recruitment in the group-average of ASD children may be greater individual variability in fMRI datasets due to variable head motion. Thus, we performed a secondary analysis of sub-samples from both groups (each N = 8) comprising subjects with less than 1mm of translational displacement in all directions. Separate two sample t-tests indicated that the maximum translational displacement did not differ between the sub-samples from the groups in any direction (X Translation – ASD: M = .16mm, SD = .13mm; Controls: M = .11mm, SD = .06mm; Y Translation – ASD: M = .45mm, SD = .21mm; Controls: M = .32mm, SD = .29mm; Z Translation – ASD: M = .53mm, SD = .28mm; Controls: M = .52; SD = .31mm) (ps > .34). In addition, age in years (ASD: M = 10.34, SD = 1.39; Controls: M = 10.81, SD = 1.62), IQ (ASD: M = 117.80, SD = 14.92; Controls: M = 111.71, SD = 13.81), accuracy (EFT - ASD: M = 63.03, SD = 13.14; Controls: M = 66.63, SD = 12.10; MATCH - ASD: M = 89.76, SD = 3.44; Controls: M = 92.39, SD = 2.58), and response time (EFT - ASD: M = 9.27s, SD = 6.65s; Controls: M = 6.17s, SD = 3.73s; MATCH - ASD: M = 1.51s, SD = .29s; Controls: M = 1.49s, SD = .20s) did not differ between these sub-samples (ps > .12). Random effects analysis (height threshold of p < .005 and cluster-level threshold of p < .05, uncorrected for multiple comparisons) revealed significant activations in all the same regions observed in each sub-sample's respective full group. However, there were additional regions - in the Control sub-sample, left inferior frontal cortex (BA 47) was activated whereas in the ASD sub-sample, bilateral middle occipital gyrus (BA 19), left occipital lobe (BA 18), and right lateral cerebellum were activated. Additional activations in sub-samples with minimal motion indicate that reduced activation in group-averages was related to greater variability among individuals with large motion. Additional activations were observed in both sub-samples, and therefore, that greater variability was not selective to the ASD group. Furthermore, the lack of additional activation in frontal regions in the ASD sub-sample indicates that reduced frontal activation in the full ASD group is not an artifact of motion-related variability.
A second reason for the absence or reduction of regional recruitment in the group-average of ASD children may be high spatial variability in regions activated at the individual level. Thus, a post-hoc subject-by-subject inspection of significant activations present in Controls but not ASD was conducted to determine whether any voxels reached the height threshold (Table 1). There were no regions significantly activated in the ASD group that were not also present in the Control group. Of the 13 control children, all recruited prefrontal cortex laterally (BA 9/46) and 11 medially (BA 8/32). In contrast, of the 13 ASD children, only six recruited it laterally and four of these six also recruited it medially. For ventral-temporal cortex, all control children activated fusiform gyrus bilaterally whereas only four ASD children activated it bilaterally and two in the right hemisphere. For parietal cortex, eight control children activated superior regions bilaterally (BA 7) and nine activated left inferior regions (BA 40). In contrast, four ASD children activated superior regions bilaterally, while three activated left inferior regions. Finally, 12 control children and seven ASD children showed bilateral occipital activation (BA 18). Absence of activation in regions in the ASD group does not appear to be a product of greater motion because ASD children with greater than 1mm of translational displacement (ASD 1, 6, 8, 9, and 13 in Table 1) showed both presence (ASD 1) and absence (ASD 13) of activation. Thus, it appears that fewer ASD than control children exhibited the activation loci observed in the control group-average. Therefore, absence of those regions in the ASD group-average resulted from a lack of recruitment in most ASD children, rather than spatial variability.
Discussion
The functional anatomy of EFT performance differed between pre-adolescent ASD children and typically developing control children. Behaviorally, the groups did not differ in accuracy, number of trials completed, or response time for the EFT or the MATCH. While EFT performance was well-matched in ASD and control children, subserving neural recruitment differed in two ways: First, control children activated left dorsolateral and medial prefrontal cortex and bilateral ventral temporal cortex. ASD children did not activate these regions reliably. Second, superior parietal and occipital cortical involvement was atypical in ASD relative to control children. These regions were recruited bilaterally in control children but unilaterally in ASD. Only one region, left dorsal premotor cortex, was activated similarly across the two groups. Therefore, despite similar accuracy and response times on the EFT, the underlying neural network differed qualitatively between ASD and control children.
The present behavioral results did not reveal superior performance on the EFT in ASD relative to controls. However, our findings are consistent with fMRI studies of EFT performance in ASD adults (Ring et al., 1999) and adolescents (Manjaly et al., 2007). One difference between fMRI studies that did not find an advantage in ASD and behavioral studies that did is that behavioral studies typically administer the task in a paper-pencil format - the simple target shape is presented briefly and then removed before individuals attempt to trace it within the complex probe figure. In contrast, fMRI studies present the simple target and complex probe figures simultaneously, perhaps providing more opportunity for visual-spatial comparison or reducing working memory demands in ways that may be selectively advantageous for controls. Thus, scanner-friendly adaptations of the task may not optimize conditions under which controls show poorer performance relative to individuals with ASD. Task format may not be the only reason for the lack of behavioral group differences because the present finding is consistent with at least one paper-pencil study of the EFT (Brian & Bryson, 1996). However, that study included lower-functioning children with ASD whereas fMRI studies typically include higher-functioning ASD subjects because of procedural demands of performing in the MRI environment. Indeed, the current study included ASD children with relatively high IQs. While it is possible that the high-functioning ASD groups used in imaging studies perform similarly to controls on the EFT, there is at least one other paper-pencil study of high-functioning subjects that found a behavioral advantage in ASD (Jolliffe & Baron-Cohen, 1997). Thus, whether the nature of task presentation or the intellectual status of ASD subjects determines level of EFT performance remains ambiguous. Nevertheless, for interpretation of functional brain imaging results, a lack of behavioral difference is advantageous because it allows elimination of variability in speed and/or accuracy as the source of differences in brain recruitment.
Despite a lack of behavioral support, group differences in prefrontal recruitment suggest a processing style that is consistent with weak central coherence in ASD. Normative perception is known to be biased towards preferential processing of global form (Kimchi, 1992) and its encoding and inhibition is thought to be reflected in lateral and medial prefrontal activation, respectively (Lux et al., 2004; Weissman et al., 2003). In the present study, activation of those regions in control children, but not in ASD, suggests that global form of the complex visual figure was processed to a greater extent by control than ASD children. The medial prefrontal activation observed here, in the anterior cingulate extending dorsally, was also involved during perception of local aspects of hierarchical stimuli (Lux et al., 2004; Weissman et al., 2003) and is posited to reflect processes involved in suppression of the global perceptual bias. Lack of that activation in ASD children suggests a reduced need to suppress a global bias, consistent with the weak central coherence view. Further, similar to the present findings in control children, past EFT studies reported involvement of dorsolateral prefrontal cortex (Ring et al., 1999) in regions known to represent visual-spatial working memory (Passingham & Sakai, 2004). However, unlike its right hemisphere locus in adults, the homologous region in the left hemisphere was activated by control children in the present study, consistent with the use of verbal working memory (Wager & Smith, 2003). Thus, control children may have used verbal strategies for maintaining and manipulating the complex visual figure in working memory during the EFT. Indeed, in studies of other tasks requiring executive control, children activated left rather than right ventrolateral prefrontal cortex seen in adults, suggesting the greater use of a verbal strategy (Bunge et al., 2002). Lack of dorsolateral prefrontal involvement in ASD may indicate a reduced need for a verbal strategy to represent the global form during EFT performance, further suggesting a processing style consistent with weak central coherence. Despite these prefrontal cortical differences between the groups, left dorsal premotor cortex associated with response planning (Picard & Strick, 2001) was recruited to a similar degree. Thus, atypical prefrontal but similar premotor cortical involvement in ASD children suggests that target local shapes were identified without consideration of the global form of the complex figure.
Temporo-parieto-occipital contributions to resolving the EFT were reduced in ASD. First, parietal involvement unilaterally in ASD and bilaterally in controls suggests differences in spatial attentional processes between the groups. Left superior parietal involvement, observed in both ASD and control groups, has been noted by studies that selectively visualized disembedding of local shapes on the EFT (Manjaly et al., 2007; Manjaly et al., 2003). Additional recruitment of right superior parietal cortex in controls may reflect processes of shifting selective attention (Corbetta et al., 2000; Corbetta et al., 1995) and lack of this activation in ASD children suggests a reduced involvement of those processes. Moreover, ASD children engaged regions involved in spatial attentional processes to a lesser extent as inferior parietal activation was observed in control, but not ASD children. Second, reduced ventral-temporal and occipital cortical involvement in ASD relative to control children suggests decreased reliance on visual-spatial analytic processes. Ventral-temporal activation was extensive in control children, including bilateral parahippocampal regions typically activated during spatial analysis (Epstein & Kanwisher, 1998) and lateral fusiform gyri typically activated during object processing (Grill-Spector, 2003). Furthermore, control children activated primary visual cortices implicated in low-level figure-ground segregation (Heinen et al., 2005; Skiera et al., 2000) bilaterally, consistent with past EFT findings in adult controls (Ring et al., 1999). Reduced involvement of left occipital regions in ASD relative to control children suggests less reliance on lower-level visual processing. Contrary to past EFT imaging studies, however, we did not find greater right occipital recruitment in ASD than control subjects (Manjaly et al., 2007; Ring et al., 1999). Taken together, reduced engagement of temporo-parieto-occipital cortices involved in visual-spatial attention and analysis suggests a decreased reliance on these processes in ASD relative to control children.
It is noteworthy that behavioral performance and neural activations appeared to be more variable in the ASD group. Specifically, examination of Figure 2 indicates that response time and number of trials completed was more variable in ASD than control children and suggests a wider range of abilities in this group. In addition, examination of Table 1 suggests greater variability in regional recruitment for ASD children. However, regional recruitment does not appear to relate systematically to performance. For instance, ASD subject 2 had better performance (high number of completed trials) and activated all 5 regions, while ASD subject 11 had better performance but activated only 1 of the 5 regions noted. Additionally, while ASD subject 12 had poorer performance (low number of completed trials) and only activated 2 of the regions, ASD subject 4 had poorer performance and activated 4 of the 5 regions. Perhaps individual variability in regional activation relates to other behavioral characteristics such as symptom expression rather than EFT performance. Future studies should examine this question by including samples larger than that in the present study.
Our study is the first to image EFT performance in pre-adolescent children and thus provides two insights about developmental differences in visual perceptual processing: First, the pattern of prefrontal activation in control children differed from past findings with adults and adolescents. Activation of the anterior cingulate, thought to reflect suppression of processing global form, was observed in children in the present study but not in adults (Manjaly et al., 2003; Ring et al., 1999) or adolescents (Manjaly et al., 2007) in past studies. Relative to adults, children have more difficulty ignoring global distracters on hierarchical tasks (Mondloch et al., 2003) and, by extension, may have found it more effortful to suppress processing of the global form of the complex figures in the present study. Further, as discussed earlier, dorsolateral prefrontal activation was lateralized to the left in the control children in our study but to the right in other studies of adults and adolescents, perhaps reflecting greater use of verbal strategies in children (Bunge et al., 2002). Second, the bilateral pattern of temporo-parieto-occipital involvement in control children is consistent with incomplete lateralization of local/global processing using hierarchical stimuli in children (Moses et al., 2002). In contrast, activation of those regions during mature local and global processing was lateralized to the left and right hemisphere, respectively (Fink et al., 1996, 1997; Robertson & Lamb, 1991; Weissman & Woldorff, 2005). Thus, immature local perceptual processing involves differences in both frontal and posterior cortical recruitment relative to adults.
In sum, the current findings reveal a neural basis for weak central coherence as a processing style in ASD children. Our observation of reduced prefrontal involvement in ASD is consistent with the view that weak “top-down control” promotes weak central coherence in ASD (Belmonte & Yurgelun-Todd, 2003; Frith, 2004; Happe & Frith, 2006). In addition, our findings suggest that reduced reliance on visual-spatial attentional and analytic processes mediated by temporo-parieto-occipital cortices also contribute to that processing style. However, it is a matter of debate whether perceptual processing in ASD is characterized by reduced global or enhanced local processing (Happe & Frith, 2006; Mottron et al., 2003). This cannot be resolved using the EFT because performance involves an inherent trade-off between processing at the global (i.e., how relatively weak the gestalt is) and the local level (i.e., how salient the part is) (Happe & Frith, 2006). Enhanced local processing may be attained by drawing upon visual-spatial attentional and analytic resources to a greater extent. The neural correlate of such a process ought to be greater reliance on temporo-parieto-occipital regions; however, our findings show reduced reliance on those regions in ASD. Alternatively, enhanced local processing may be accomplished by more automatic visual-spatial processing. The neural correlate of this process ought to be reduced reliance on temporo-parieto-occipital engagement, a finding we observed in ASD. Thus, our findings lead to the hypothesis that a reduced need to suppress a global bias in ASD is coupled with greater automaticity in visual-spatial processing in ASD. Future behavioral and functional imaging studies ought to test this hypothesis.
Acknowledgments
This research study was funded in part by a grant from the Frederick and Elizabeth Singer Foundation to Children's National Medical Center, a grant from the National Alliance for Autism Research to CJV, and a General Clinical Research Center grant from the National Institutes of Health to Children's National Medical Center (MO1-RR020359-03). Special thanks to John VanMeter, Laura Girton, Anna Scozzofava, and Lauren Kaplan for technical assistance and imaging data collection and to Antoinette Della Rosa, Joette James and Sara McCracken for diagnostic and cognitive testing. Jennifer Foss-Feig is now at the Department of Psychology and Human Development, Vanderbilt University. Lisa Gilotty completed portions of this work while at the Center for Autism Spectrum Disorders, Children's National Medical Center. Preliminary analysis of these data was presented at the 2006 Annual Meeting of the Society for Neuroscience in Atlanta, Georgia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ADI-R: social score M = 21.4, SD = 3.9; communication score M = 17.45, SD = 3.3; restricted/repetitive behaviors M = 7.7, SD = 2.1. ADOS-G: social score M = 8.3, SD = 2.4; communication score M = 4.3, SD = 1.5
References
- A. P. A. Diagnostic and statistical manual for mental disorders. 4th edition. American Psychiatric Association; Washington, DC: 2000. text revision. [Google Scholar]
- Belmonte MK, Yurgelun-Todd DA. Functional anatomy of impaired selective attention and compensatory processing in autism. Brain Res Cogn Brain Res. 2003;17(3):651–664. doi: 10.1016/s0926-6410(03)00189-7. [DOI] [PubMed] [Google Scholar]
- Berl MM, Vaidya CJ, Gaillard WD. Functional imaging of developmental and adaptive changes in neurocognition. Neuroimage. 2006;30(3):679–691. doi: 10.1016/j.neuroimage.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Blanca MJ, Zalabardo C, Garcia-Criado F, Siles R. Hemispheric differences in global and local processing dependent on exposure duration. Neuropsychologia. 1994;32(11):1343–1351. doi: 10.1016/0028-3932(94)00067-0. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J, Valabregue R, Poline J. Region of interest analysis using an SPM toolbox (abstract) Neuroimage. 2003;16(Supplemental) (CD-ROM) [Google Scholar]
- Brian JA, Bryson SE. Disembedding performance and recognition memory in autism/PDD. J Child Psychol Psychiatry. 1996;37(7):865–872. doi: 10.1111/j.1469-7610.1996.tb01482.x. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JD. Immature frontal lobe contributions to cognitive control in children: Evidence from fMRI. Neuron. 2002;33(2):301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo J, Mitchell DW, Coldren JT, Freeseman LJ. Individual differences in infant visual attention: Are short lookers faster processors or feature processors? Child Dev. 1991;62(6):1247–1257. [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat Neurosci. 2000;3(3):292–297. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL, Miezin FM, Petersen SE. Superior parietal cortex activation during spatial attention shifts and visual feature conjunction. Science. 1995;270(5237):802–805. doi: 10.1126/science.270.5237.802. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Zarahn E, Aguirre GK, Shin RK, Auerbach P, Detre JA. The effect of pacing of experimental stimuli on observed functional MRI activity. Neuroimage. 1997;6(2):113–121. doi: 10.1006/nimg.1997.0281. [DOI] [PubMed] [Google Scholar]
- Epstein R, Kanwisher N. A cortical representation of the local visual environment. Nature. 1998;392(6676):598–601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- Fink GR, Halligan PW, Marshall JC, Frith CD, Frackowiak RS, Dolan RJ. Where in the brain does visual attention select the forest and the trees? Nature. 1996;382(6592):626–628. doi: 10.1038/382626a0. [DOI] [PubMed] [Google Scholar]
- Fink GR, Halligan PW, Marshall JC, Frith CD, Frackowiak RS, Dolan RJ. Neural mechanisms involved in the processing of global and local aspects of hierarchically organized visual stimuli. Brain. 1997;120(10):1779–1791. doi: 10.1093/brain/120.10.1779. [DOI] [PubMed] [Google Scholar]
- Frith C. Is autism a disconnection disorder? Lancet Neurol. 2004;3(10):577. doi: 10.1016/S1474-4422(04)00875-0. [DOI] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K. The neural basis of object perception. Curr Opin Neurobiol. 2003;13(2):159–166. doi: 10.1016/s0959-4388(03)00040-0. [DOI] [PubMed] [Google Scholar]
- Happe F, Frith U. The weak coherence account: Detail-focused cognitive style in autism spectrum disorders. J Autism Dev Disord. 2006;36(1):5–25. doi: 10.1007/s10803-005-0039-0. [DOI] [PubMed] [Google Scholar]
- Heinen K, Jolij J, Lamme VA. Figure-ground segregation requires two distinct periods of activity in V1: A transcranial magnetic stimulation study. Neuroreport. 2005;16(13):1483–1487. doi: 10.1097/01.wnr.0000175611.26485.c8. [DOI] [PubMed] [Google Scholar]
- Jolliffe T, Baron-Cohen S. Are people with autism and Asperger syndrome faster than normal on the embedded figures test? J Child Psychol Psychiatry. 1997;38(5):527–534. doi: 10.1111/j.1469-7610.1997.tb01539.x. [DOI] [PubMed] [Google Scholar]
- Kimchi R. Primacy of wholistic processing and global/local paradigm: A critical review. Psychol Bull. 1992;112(1):24–38. doi: 10.1037/0033-2909.112.1.24. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Forssberg H, Westerberg H. Increased brain activity in frontal and parietal cortex underlies the development of visuospatial working memory capacity during childhood. J Cogn Neurosci. 2002;14(1):1–10. doi: 10.1162/089892902317205276. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr., Leventhal BL, DiLavore PC, et al. The Autism Diagnostic Observation Schedule-Generic: A standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lux S, Marshall JC, Ritzl A, Weiss PH, Pietrzyk U, Shah NJ, et al. A functional magnetic resonance imaging study of local/global processing with stimulus presentation in the peripheral visual hemifields. Neuroscience. 2004;124(1):113–120. doi: 10.1016/j.neuroscience.2003.10.044. [DOI] [PubMed] [Google Scholar]
- Manjaly ZM, Bruning N, Neufang S, Stephan KE, Brieber S, Marshall JC, et al. Neurophysiological correlates of relatively enhanced local visual search in autistic adolescents. Neuroimage. 2007;35(1):283–291. doi: 10.1016/j.neuroimage.2006.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjaly ZM, Marshall JC, Stephan KE, Gurd JM, Zilles K, Fink GR. In search of the hidden: An fMRI study with implications for the study of patients with autism and with acquired brain injury. Neuroimage. 2003;19(3):674–683. doi: 10.1016/s1053-8119(03)00095-8. [DOI] [PubMed] [Google Scholar]
- Mondloch CJ, Geldart S, Maurer D, de Schonen S. Developmental changes in the processing of hierarchical shapes continue into adolescence. J Exp Child Psychol. 2003;84(1):20–40. doi: 10.1016/s0022-0965(02)00161-3. [DOI] [PubMed] [Google Scholar]
- Moses P, Roe K, Buxton RB, Wong EC, Frank LR, Stiles J. Functional MRI of global and local processing in children. Neuroimage. 2002;16(2):415–424. doi: 10.1006/nimg.2002.1064. [DOI] [PubMed] [Google Scholar]
- Mottron L, Burack JA, Iarocci G, Belleville S, Enns JT. Locally oriented perception with intact global processing among adolescents with high-functioning autism: Evidence from multiple paradigms. J Child Psychol Psychiatry. 2003;44(6):904–913. doi: 10.1111/1469-7610.00174. [DOI] [PubMed] [Google Scholar]
- Passingham D, Sakai K. The prefrontal cortex and working memory: Physiology and brain imaging. Curr Opin Neurobiol. 2004;14(2):163–168. doi: 10.1016/j.conb.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Patton GC, Viner R. Pubertal transitions in health. Lancet. 2007;369(9567):1130–1139. doi: 10.1016/S0140-6736(07)60366-3. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL. Imaging the premotor areas. Curr Opin Neurobiol. 2001;11(6):663–672. doi: 10.1016/s0959-4388(01)00266-5. [DOI] [PubMed] [Google Scholar]
- Ring HA, Baron-Cohen S, Wheelwright S, Williams SC, Brammer M, Andrew C, et al. Cerebral correlates of preserved cognitive skills in autism: A functional MRI study of embedded figures task performance. Brain. 1999;122(Pt 7):1305–1315. doi: 10.1093/brain/122.7.1305. [DOI] [PubMed] [Google Scholar]
- Robertson LC, Lamb MR. Neuropsychological contributions to theories of part/whole organization. Cognit Psychol. 1991;23(2):299–330. doi: 10.1016/0010-0285(91)90012-d. [DOI] [PubMed] [Google Scholar]
- Shah A, Frith U. An islet of ability in autistic children: A research note. J Child Psychol Psychiatry. 1983;24(4):613–620. doi: 10.1111/j.1469-7610.1983.tb00137.x. [DOI] [PubMed] [Google Scholar]
- Skiera G, Petersen D, Skalej M, Fahle M. Correlates of figure-ground segregation in fMRI. Vision Res. 2000;40(15):2047–2056. doi: 10.1016/s0042-6989(00)00038-9. [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: A meta-analysis. Cogn Affect Behav Neurosci. 2003;3(4):255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Giesbrecht B, Song AW, Mangun GR, Woldorff MG. Conflict monitoring in the human anterior cingulate cortex during selective attention to global and local object features. Neuroimage. 2003;19(4):1361–1368. doi: 10.1016/s1053-8119(03)00167-8. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Gopalakrishnan A, Hazlett CJ, Woldorff MG. Dorsal anterior cingulate cortex resolves conflict from distracting stimuli by boosting attention toward relevant events. Cereb Cortex. 2005;15(2):229–237. doi: 10.1093/cercor/bhh125. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Woldorff MG. Hemispheric asymmetries for different components of global/local attention occur in distinct temporo-parietal loci. Cereb Cortex. 2005;15(6):870–876. doi: 10.1093/cercor/bhh187. [DOI] [PubMed] [Google Scholar]
- Witkin HA, Oltman PK, Rashkin E, Karp SA. A manual for the embedded figures test. Consulting Psychologists Press; Mountain View, CA: 1971. [Google Scholar]


