Table 1.
Synthesis of carbazoles and analoguesa
| entry | substrate | aryl triflate | CsF
(equiv) |
product | % isolated
yield |
|---|---|---|---|---|---|
| 1 |
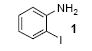
|
1a | 3.0 |
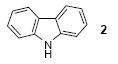
|
77 |
| 2 | 1 | 1a | 5.0 |
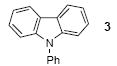
|
66b |
| 3 | 1 | 1b | 3.0 |
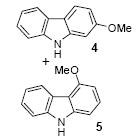
|
61
(5:1) |
| 4 |
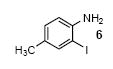
|
1c | 3.0 |
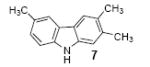
|
68 |
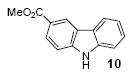
| 5 | 6 | 1a | 3.0 |
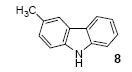
|
69 |
| 6 |
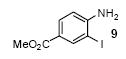
|
1a | 3.0 |
|
68 |
| 7 |
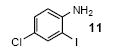
|
1a | 3.0 |
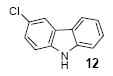
|
72 |
| 8 |
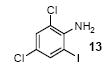
|
1a | 3.0 |
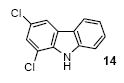
|
87 |
| 9 | 13 | 1c | 3.0 |
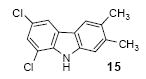
|
85 |
| 10 |
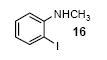
|
1a | 3.0 |
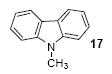
|
82 |
| 11 | 16 | 1c | 3.0 |
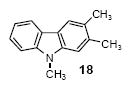
|
71 |
| 12 |
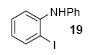
|
1a | 3.0 |
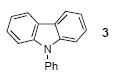
|
76c |
| 13 |
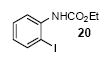
|
1a | 3.0 |
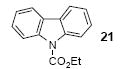
|
85 |
| 14 | 20 | 1c | 3.0 |
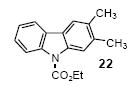
|
85 |
| 15 |
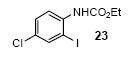
|
1a | 3.0 |
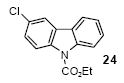
|
86 |
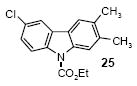
| 16 | 23 | 1c | 3.0 |
|
85 |
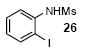
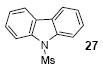
| 17 |
|
1a | 3.0 |
|
85 |
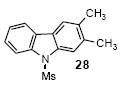
| 18 | 26 | 1c | 3.0 |
|
85 |
| 19 | 26 |
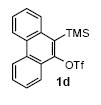
|
3.0 |
|
62 |
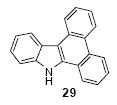
| 20 |
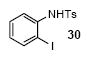
|
1a | 3.0 |
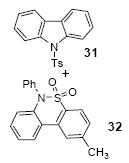
|
83
(1:1) |
| 21 |
|
1a | 3.0 |
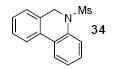
|
66 |
| 22 |
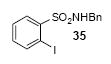
|
1a | 3.0 |
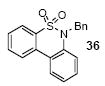
|
62 |
Reaction conditions: 0.25 mmol of aryl iodide are allowed to react with 1.1 equiv of the aryl triflate and the number of equiv of CsF shown in the table in 4.0 mL of MeCN as the solvent at room temperature for 10 h, followed by the addition of 5 mol % Pd(OAc)2 and 10 mol % PCy3 and heating for 1 d at 100 °C .
2.4 Equiv of aryl triflate were emp loyed and the reaction was run at room temperature for 2 d, followed by the addition of 5 mol % Pd(OAc)2 and 10 mol % PCy3 and heating for 1 d at 100 °C.
The reaction was run at room temperature for 1.5 d, followed by the addition of 5 mol % Pd(OAc)2 and 10 mol % PCy3 and heating for 1 d at 100 °C.
