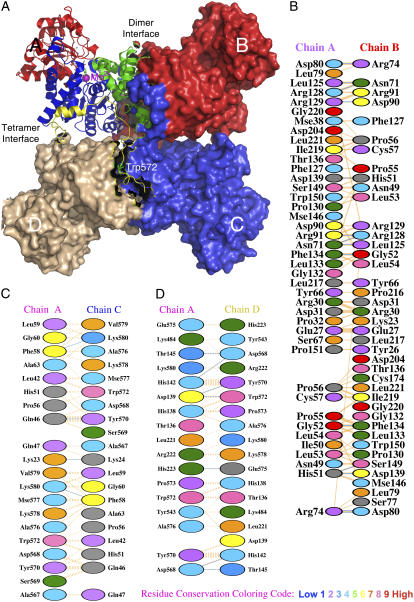
FIGURE 1.
Overall quaternary structure and the interfacial regions of pigeon c-NADP-ME. (A) The tetrameric pigeon cytosolic ME (pdb code: 1GQ2) is shown as a surface model that clearly illustrates the double dimer structure of the enzyme. One of the monomers located at the upper left corner is shown in ribbon diagram and illustrates the four structural domains: domain A in green, domain B in blue, domain C in red, and domain D in yellow. In the active site, the transition state analog oxalate (in green) and coenzyme NADP+ are shown in bond form, and the manganese ion is shown as a purple sphere. The unique Trp-572 is shown in bond form within domain D. The figure was generated using PyMOL (37). (B–D) Interfacial regions of c-NADP-ME. (B) A-B interface interactions. (C) A-C interface interactions. (D) A-D interface interactions. Panels B–D were generated using PDBsum (http://www.ebi.ac.uk/thornton-srv/databases/pdbsum/). Hydrogen bonds are shown in cyan, and nonbonded interactions are shown in orange. The width of the striped line is proportional to the number of atomic contacts. The interfacial amino acid residues are colored by residue conservation as shown in the bottom of the figure. Trp-572 is a highly conserved residue among all ME.