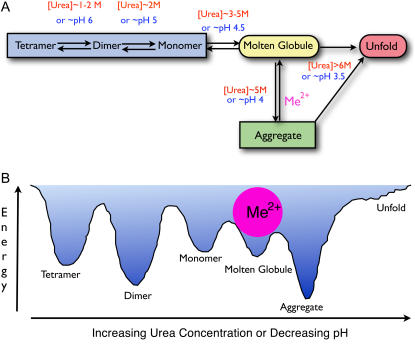
FIGURE 9.
Quaternary structural changes of ME. (A) A working model for the structural changes of ME in urea solution or in an acidic environment. The enzyme exists as a tetramer in buffer at neutral pH and starts to dissociate into dimers at 1–2 M urea or at pH 6 and then finally into monomers at 2 M urea or pH 5. A further increase of urea concentration to 3–5 M or decrease in the pH to 4.5 causes an overall structural change to a molten globule state, which has the propensity to aggregate that shunts the enzyme into a divergent misfolding pathway (off-pathway). Metal ions at this point can play a pivotal role in directing the correct refolding (on-pathway) when the structure of ME is perturbed to the folded-misfolded crossroads. (B) Hypothetical potential energy changes of the enzyme in increasing urea solution or different pH environments. Divalent metal ions are shown to bind with the molten globule state and thus prevent aggregation of the enzyme.