Fig. 7.
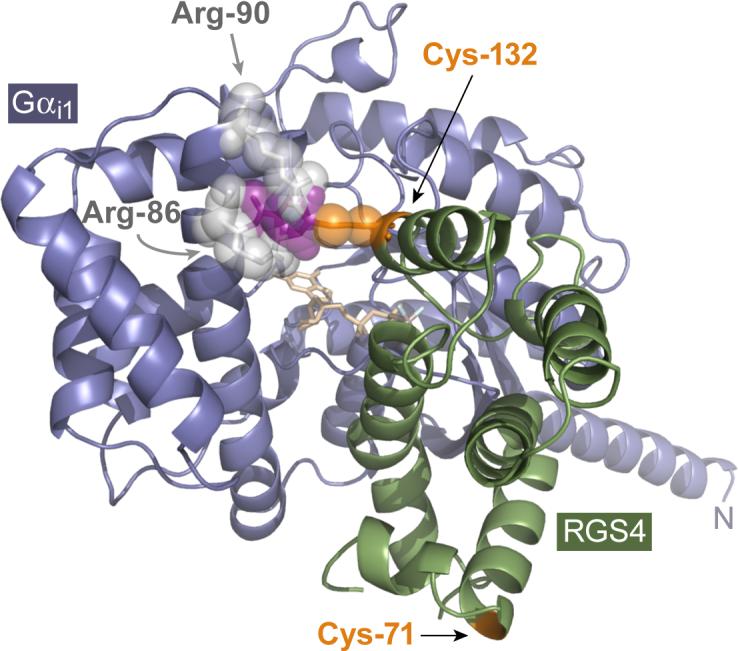
Proposed model for RGS4 inhibition by CCG-4986. The presence of the cysteine-132 residue of RGS4 is clearly necessary for the inhibitory action of CCG-4986. While cysteine-132 is not critical for the RGS4/Gα interaction per se (i.e., its conversion to glutamate does not eliminate GAP activity [Fig. 2] nor Gα association [Figs. 3 & 4]), the known high-resolution structure of the RGS4/Gαi1 complex [18] suggests that a small moiety (purple) covalently coupled to Cys-132 (orange) would result in steric hindrance with the Arg-86 and Arg-90 (light gray) of the all-helical domain of Gαi1 (steel blue). The Cα ribbon trace of RGS4 is illustrated in green; GDP bound within the G-protein is colored in wheat, with the aluminum tetrafluoride ion in light blue and gray.
