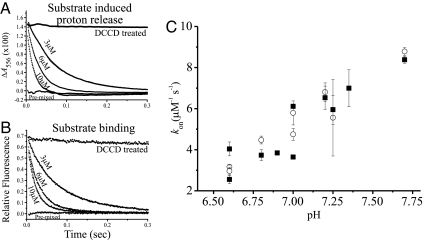
Fig. 4.
Parallel measurement of the kinetics of substrate-binding and substrate-induced proton release. (A) Stopped-flow measurements of proton release: 3 μM EmrE in a solution of 0.15 M NaCl, 0.08% DDM buffered with 100 μM phenol red (pH 7.0) was mixed with 3, 6, and 10 μM TPP+. Shown also is protein inhibited with DCCD (pretreated with 500 μM, 60 min at room temperature) and protein mixed with TPP+ before the experiment. Absorption changes at 556 nm were collected. (B) Same solutions as in A, measured for tryptophan fluorescence changes at 280-nm excitation and >320-nm emission. The data on A and B are an average of 5–10 repeats, fitted to an exponential equation. (C) Summary of the kon values obtained from measurements like those presented in A and B for 1.5 or 3 μM EmrE at different pH values. The kon was calculated by using the slope of the linear fit of the kobs against [TPP+] according to Eq. 1. Squares are kon obtained from substrate-induced proton release measurements, and open circles are kon obtained from the same solutions measured for substrate binding by using tryptophan fluorescence changes.