Abstract
Increased sympathetic drive to the heart worsens prognosis in heart failure, but the level of cardiac sympathetic nerve activity (CSNA) has been assessed only by indirect methods, which do not permit testing whether its control by arterial baroreceptors is defective. To do this, CSNA was measured directly in 16 female sheep, 8 of which had been ventricularly paced at 200–220 beats/min for 4–6 weeks, until their ejection fraction fell to between 35 and 40%. Recording electrodes were surgically implanted in the cardiac sympathetic nerves and after 3 days’ recovery, responses to intravenous phenylephrine and nitroprusside infusions were measured in conscious sheep. Electrophysiological recordings showed that resting CSNA (bursts/100 heart beats) was significantly elevated in heart failure sheep (89±3) compared with normal animals (46±6, P<0.001). This increased CSNA was not accompanied by any increase in the low frequency power of heart rate variability. The baroreceptor- heart rate reflex was significantly depressed in heart failure (maximum gain −3.29±0.56 vs. −5.34±0.66 beats/min/mmHg in normal animals), confirming published findings. In contrast, the baroreflex control of CSNA was undiminished (maximum gain in heart failure −6.33±1.06, vs. −6.03±0.95 %/mmHg in normal sheep). Direct recordings in a sheep model of heart failure thus show that resting CSNA is strikingly increased, but this is not due to defective control by arterial baroreceptors.
Keywords: Baroreceptors, cardiac sympathetic nerve activity, heart failure, pacing, spectral analysis
Introduction
Heart failure (HF) is characterized by activation of the sympathetic nervous system as demonstrated in patients by increases in circulating norepinephrine (44), total and regional norepinephrine spillover (19) and muscle sympathetic nerve activity (MSNA) (17). In animal models of both pacing- and infarction-induced HF, it has also been demonstrated by direct nerve recording that renal sympathetic nerve activity (RSNA) is increased (9, 28), as is renal NE spillover in humans with HF (19).
The mechanisms that cause the sympathetic activation in HF are not well understood. This is particularly true for the outflow to the heart as there are no studies in which cardiac sympathetic nerve activity (CSNA) has been recorded directly in HF. Understanding the factors leading to the increased CSNA in HF is particularly important because it is the increase in CSNA that leads to arrhythmias and sudden death. The detrimental effect of increased CSNA in HF is shown by the strong inverse correlation between cardiac norepinephrine spillover and prognosis (23) and by the beneficial action of β-adrenergic blockers on survival (38).
Studies of cardiac norepinephrine spillover, an indirect, steady state method, indicate that it is increased more than spillover from other organs in patients with HF (19), and that the cardiac increase occurs earlier in the disease process than in other organs (41). These data imply that in HF there is a preferential activation of CSNA, exposing the heart to higher levels of activation, and for a longer period, than to other organs. On the other hand, changes occur to the cardiac sympathetic nerves in HF which confound the interpretation of indirect functional measures. The sympathetic innervation of the heart progressively rarefies during the development of HF (20) and norepinephrine uptake may be impaired (12, 41). Consequently, the relationship of these indirect measures to the neural drive received by the heart remains unclear.
The arterial baroreflex is a potent inhibitory reflex with dominant control over resting sympathetic nerve activity, and its role in the activation of non-cardiac sympathetic outflow in HF has been studied extensively. In HF patients, the sensitivity of the baroreflex control of MSNA has been reported to be reduced (17, 25, 31), with similar changes in the baroreflex control of RSNA reported in conscious rabbits with pacing-induced HF (26) and in rats with HF induced by coronary occlusion (9). Other studies, however, have found preserved arterial baroreflex control of MSNA in HF patients (6) and in a recent review it has been argued that the arterial baroreflex control of sympathetic nerve activity is not desensitised even in advanced HF (15). In experimental animals, preserved baroreflex control of RSNA was found in anaesthetised dogs with HF induced by pacing or an AV fistula (7, 48). Desensitised arterial baroreflex control of heart rate has been reported widely in HF (11, 14, 17), so it seemed reasonable to propose that the reduced HR changes in response to pressor and depressor stimuli depended not only on the well established attenuation of parasympathetic control (3, 10, 11, 40), but also on impaired cardiac sympathetic responses. Without direct recordings of CSNA in HF, it remained unknown whether the arterial baroreflex control of CSNA is desensitized in this state.
To determine the level of sympathetic drive to the heart in HF we directly recorded CSNA in conscious normal healthy sheep and in sheep with HF induced by rapid ventricular pacing. These measures were compared side by side with a commonly used indirect index of sympathetic drive, the low frequency component of heart rate variability. We then assessed the arterial baroreflex control of CSNA to test whether it was desensitised in HF. Importantly the studies were conducted on untreated, conscious animals, thus avoiding the confounding actions of drugs or of anesthesia, which has a particularly potent depressive effect on CSNA (32).
Materials and Methods
Adult merino ewes were housed in individual metabolism cages in association with other sheep. Experiments were conducted on conscious sheep standing in their home cage and were not started until they were accustomed to laboratory conditions and human contact. Sheep were fed a diet of oaten chaff (800 g/day), and water was offered ad libitum. All experiments were approved by the Animal Experimentation Ethics Committee of the Howard Florey Institute.
Surgical Procedures
Prior to the studies, sheep underwent 2 or 3 aseptic surgical procedures, each separated by 2 weeks recovery. Anesthesia was induced with intravenous sodium thiopental (15mg/kg) and following intubation was maintained with 1.5–2.0% isoflurane/O2. In the first stage 16 sheep were prepared with a carotid arterial loop. For induction of HF in 8 sheep, a pacemaking lead (Medtronic Inc., Minneapolis, MN, USA) was inserted under fluoroscopic guidance into the apex of the right ventricle via the right jugular vein. The lead was exteriorized on the neck and connected to an external pacemaker. At experiment, sheep in HF weighed 43.5 ±1.6 kg and normal sheep weighed 40.8 ± 2.0 kg.
In a further operation, intrafascicular needle electrodes were implanted in the left cardiothoracic nerves as described previously (47). The left cardiothoracic nerves innervate the left and right ventricular muscle, the left atrium and septum, as well as the sinoatrial and atrioventricular nodes (34, 46). Briefly, an incision was made above the 4th rib, the periosteum was opened and the rib removed. The thoracic cardiac nerves were identified and the facia over the nerves was removed. Up to 5 electrodes were pushed obliquely through the nerve sheath, ensuring that the tip was positioned in the center of the nerve (47). Electrodes were fixed in place with cyanoacrylate glue, the implantation site was covered with a layer of Kwik-Sil (WPI, Glen Waverly, Victoria, Australia) and the wires were exteriorised next to the sutured wound. A stainless steel suture looped through the skin was used as an earth. Animals were treated with antibiotics (Ilium Propen; procaine penicillin, Troy Laboratories Ptd Ltd, Smithfield, NSW, Australia) at the start of surgery and then for 2 days postoperatively. Post-surgical analgesia was maintained with intramuscular injection of flunixin meglumine (1 mg/kg) (Troy Laboratories) at the start of surgery, then 4 and 16 hours post-surgery. To minimise any effect of surgical stress experiments were not started until the third day after implantation of the electrodes. On the day before implantation of recording electrodes, cannulae were implanted into the carotid artery loop and a jugular vein for the measurement of arterial pressure and for intravenous infusion (47).
Experimental protocols
The development of HF was assessed by measurement of ejection fraction using short axis M-wave echocardiography on conscious sheep lying on their right side. Following placement of ventricular pacing leads, a basal measurement was made before the start of ventricular pacing, at 200–220 beats/min. Echocardiography was performed weekly with the pacing switched off. Sheep were considered to be in HF when ejection fraction had fallen to <40%. All experiments were conducted with the pacing switched off.
CSNA was recorded differentially between the pair of electrodes with the best signal-to-noise ratio. The signal was amplified (× 100,000) and filtered (bandpass 300–1,000 Hz), displayed on an oscilloscope, and passed through an audio amplifier and loud speaker. Sympathetic nerve activity (5000 Hz) and arterial blood pressure (100 Hz) were recorded on computer using a CED micro 1401 interface and Spike 2 software (Cambridge Electronic Design, UK).
At 3 days after surgical implantation of cardiac sympathetic nerve electrodes, a 5 minute recording of resting CSNA and arterial pressure was made in conscious sheep in the normal state and in HF. Following this, baroreflex curves were generated by measuring the CSNA and heart rate responses to increases and decreases in arterial pressure induced by intravenous administration of incremental doses of phenylephrine and sodium nitroprusside. Phenylephrine hydrochloride (33, 67, 133 and 330 mg/min, Neo-synephrine, Abbot Australasia Ptd. Ltd., Kurnell, NSW, Australia) and sodium nitroprusside (42, 83, 167, and 417 mg/min; David Bull Laboratories, Mulgrave, Victoria, Australia) were infused at 0.5, 1.0, 2.0, 5.0 mL/min for 1–2 min at each dose, with treatments given in random order.
Data Analysis
Data were analysed on a beat-to-beat basis using custom written routines in the Spike 2 program. For each heart beat the program determined diastolic, systolic and mean arterial blood pressures, heart period (HP) and the number of discriminated spikes above threshold between the following diastolic pressures, a measure of burst size. The threshold was set just above background so that spikes from small bursts were counted. The background noise was taken as the spikes/second during the highest dose of phenylephrine when CSNA was abolished, and this was subtracted from the data collected on that day.
As a measure of CSNA, the spikes over threshold minus the background were calculated for each heart beat. Burst frequency was calculated as the percentage of heart beats that included spikes above background. The accuracy of burst determination was checked by eye over the 5 minute control data for each sheep. Post-hoc analysis of data was conducted using custom written scripts for Spike2 and data were exported to a spreadsheet for further analysis Baroreceptor relations were constructed from data collected during infusion of phenylephrine and nitroprusside. The burst size, determined as the number of discriminated spikes between successive diastolic pressures, was divided by the heart period. The relation between CSNA and diastolic blood pressure was determined because, on a beat-to-beat basis, diastolic pressure has a closer correlation to sympathetic activity than either systolic or mean arterial pressures (43). To correct for natural variation in CSNA levels between sheep, spike counts were expressed as a percentage of the mean activity recorded during the 5 min control period. The data were then sorted by diastolic pressure. The mean values of groups of 10 data points were used to plot the burst size against diastolic pressure using a four-parameter sigmoidal logistic equation to fit the data (SigmaPlot; SPSS Inc., ver. 8.0) (16). The CSNA data were normalised, using the top and bottom plateaus that represent the maximum level during sodium nitroprusside and zero activity during phenylephrine, respectively. Normalised CSNA was plotted against diastolic pressure using the four-parameter sigmoidal logistic equation. Heart rate was plotted against systolic BP because changes in vagal activity are the primary cause of changes in heart rate in response to increases and decreases in arterial pressure, and vagal tone is more closely correlated with systolic than diastolic BP. Variables from the equations of all the graphs were grouped for the normal and HF animals and average baroreflex relations plotted.
Power spectral density was calculated for both the normal and the HF sheep for the heart rate and the CSNA signals using custom written routines in Spike 2. For the CSNA signal, the raw nerve activity was full wave rectified and integrated with a time constant of 20ms. For both the heart rate and CSNA signals, the DC level was removed and the signals were re-sampled at 1 Hz. Five-minute segments of stationary data were then split into eight segments overlapping by 50%. A Hanning window was applied to each segment and the average spectral density plot was found from each segment and averaged to obtain a mean spectral density plot. Power was calculated in the band range of 0.05 to 0.15 Hz (low frequency power) and 0.20 to 0.30 Hz (mid frequency power). Power in these bands was expressed as a percentage of total power.
Data are expressed as means ± SEM and were analysed using unpaired Student’s t-tests (SigmaStat, Access Softek, ver 2.03). P<0.05 was considered statistically significant.
Results
Resting Hemodynamics and Heart Rate Variability
Left ventricular ejection fraction and fractional shortening, measured in conscious sheep by echocardiography, gradually decreased over 4–6 weeks of rapid ventricular pacing at 200–220 beats/min. At 1–2 days before implantation of cardiac sympathetic recording electrodes, HF animals had an ejection fraction of 36 ± 2 %, compared with 82 ± 3 % before pacing (P<0.001). Fractional shortening was decreased from 48 ±2 to 19±1 % (P<0.001) and there were increases in end diastolic diameter (from 3.5 ±0.1 to 4.4 ± 0.2 cm, P=0.015) and end systolic diameter (from 1.9 ± 0.1 to 3.5 ±0.2 cm, P=0.001). An increase in pericardial fluid was visible by echocardiography in 5 of the HF sheep and at surgery HF animals had 100 to 500 mL of pleural effusion. Systolic blood pressure (BP) (P=0.073), dBP (P=0.065) and mean arterial pressure (P=0.055) all tended to be lower in HF sheep, although these differences did not reach significance (Table 1). Animals in HF had a significantly higher mean HR than normal animals and lower heart rate variability, as measured by the standard deviation of the R-R interval (Table 1). Spectral analysis of heart rate variability showed no difference in the proportion of low frequency spectral power between normal and HF sheep (40.6 ± 8 % vs. 40.2 ± 6 % of total power, respectively; Figure 2), and no difference in the ratio of low frequency to mid frequency spectral power (3.4 ± 1.2 in normals vs. 3.1 ± 0.9 in HF).
Table 1.
Resting points of normal animals and animals in heart failure from 5 minutes of control data (n=8 both groups).
| Normal | Heart Failure | |
|---|---|---|
| Burst Frequency (%) | 46 ± 6 | 89 ± 3 *** |
| CSNA (% of maximum) | 22.5 ± 3.3 | 61.2 ± 6.5 *** |
| CSNA (spikes/sec) | 13.9 ± 4.2 | 44.6 ± 7.9 ** |
| Heart Rate (beats/min) | 69.9 ± 4.9 | 87.1 ± 3.1 * |
| Diastolic BP (mmHg) | 74.9 ± 2.0 | 69.4 ± 1.8 |
| Systolic BP (mmHg) | 101.5 ± 2.8 | 93.3 ± 3.2 |
| Mean Arterial Pressure (mmHg) | 83.7 ± 2.1 | 77.2 ± 2.2 |
| Pulse Pressure (mmHg) | 26.5 ± 1.4 | 24.0 ± 1.6 |
| SD of RR interval (msec) | 57.4 ± 7.5 | 32.8 ± 7.3 ** |
Abbreviations: CSNA- cardiac sympathetic nerve activity, HR- heart rate, BP blood pressure.
P<0.05,
P<0.01,
P<0.001 vs normal group.
Figure 2.
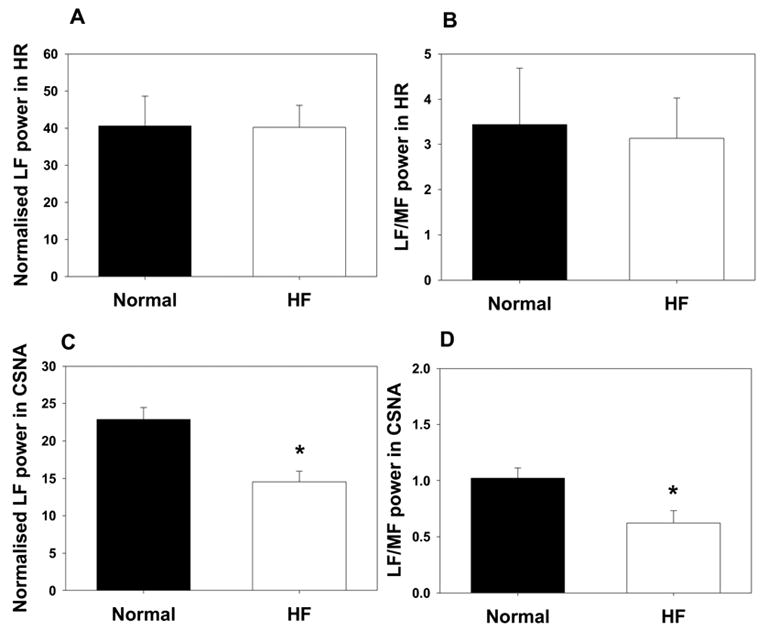
Normalised low frequency spectral power (A) and ratio of low to mid frequency spectral power (B) in heart rate (HR) in normal sheep (dark bars; n=8) and sheep in heart failure (clear bars; n=8). Panels C and D represent normalised low frequency spectral power (C) and ratio of low to mid frequency spectral power (D) in CSNA in normal sheep (dark bars; n=8) and sheep in heart failure (clear bars; n=8) indicating a decrease in power in the HF sheep. *P<0.05 compared with normal group.
CSNA measurements
Like the activity in other sympathetic nerves, CSNA occurred in arterial pulse-synchronous bursts: this was true in both normal and HF sheep (Figure 1). In 4 of the normal sheep we demonstrated that CSNA was blocked by ganglion blockade with hexamethonium, indicating that the recordings from these nerves were of post-ganglionic, efferent sympathetic nerve activity (data not shown). The resting burst incidence of CSNA in 8 HF sheep was 89 ± 3 % (range 80 to 100 %), compared with 46 ± 6 % (range 20 to 70 %) in 8 normal animals (P<0.001) (Table 1). The resting CSNA burst rate, expressed as a percentage of its maximum level during infusion of sodium nitroprusside, was also significantly higher in the HF sheep (61.2 ± 6.5%) compared with the normal sheep (22.5 ± 3.3 %) (P<0.001, n=8 in each group). Comparisons of raw nerve activity in different animals have to be interpreted cautiously because the absolute size of the signal is affected by physical factors such as nerve fascicle size and electrode placement. Nevertheless, the measured raw CSNA spike count was substantially greater in the HF sheep (44.6 ± 7.98 spikes/sec) than in normal sheep (13.9 ± 4.2 spikes/sec) (P<0.01) (Table 1). Spectral analysis of the CSNA signal showed that 22.9 ± 1.6 % of total power was present in the low frequency range in normal animals, which fell to 14.6 ± 1.35 % in the HF group (P<0.01, Figure 2). The ratio of low frequency to mid frequency power was also reduced in the HF group (1.0 ± 0.1 % in normal animals vs. 0.6 ± 0.1 % in HF; P<0.05).
Figure 1.
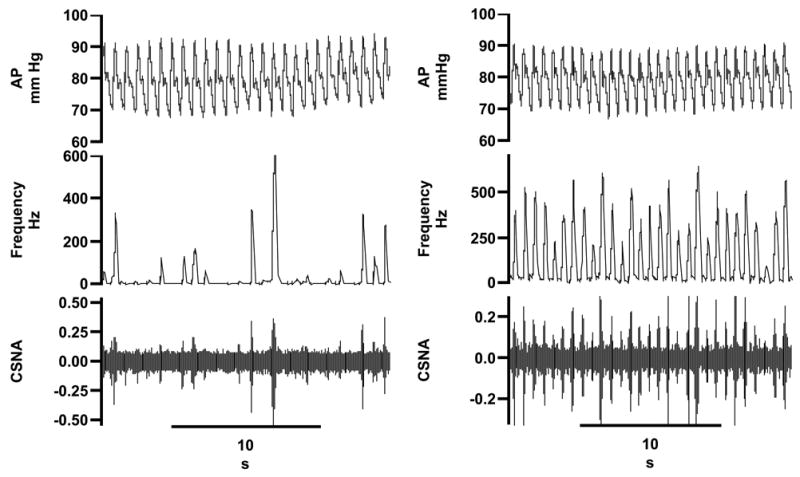
Direct recordings of cardiac sympathetic nerve activity (CSNA) and arterial pressure (AP) in a normal sheep (left panel) and in a sheep with heart failure (right panel). Traces in both panels are (top to bottom) arterial pressure, the frequency of CSNA spikes over threshold (0.2 sec bins), raw CSNA. Time in seconds (s).
Baroreflex Control of Heart Rate and CSNA
Composite baroreflex relations between HR and systolic BP in 8 normal sheep and in 8 sheep with HF are shown in Figure 3. The maximum gain of the relation in HF animals (−3.29 ± 0.56 beats/min/mmHg) was less than that in normal animals (−5.34 ± 0.66 beats/min/mmHg) (P<0.05), confirming observations in humans and other species (11, 14, 17). The upper plateau of heart rate was also significantly lower in the HF group (117.1 ± 4.3 beats/min) compared with normal sheep (162.0 ± 11.4 beats/min) ( P<0.01), whereas the bottom plateaus were similar in the two groups (Table 2). Similar findings were obtained if baroreflex relations between HR and diastolic BP were calculated. In the normal and HF groups, respectively, the maximum gains were −5.20 ± 0.76 and −2.26 ± 0.36 beats/min/mmHg (P<0.05), the upper plateaus were 163.0 ± 8.5 and 118.8 ± 5.6 beats/min (P<0.001) and the bottom plateaus were 49.5 ± 5.7 and 51.8 ± 3.1 beats/min.
Figure 3.
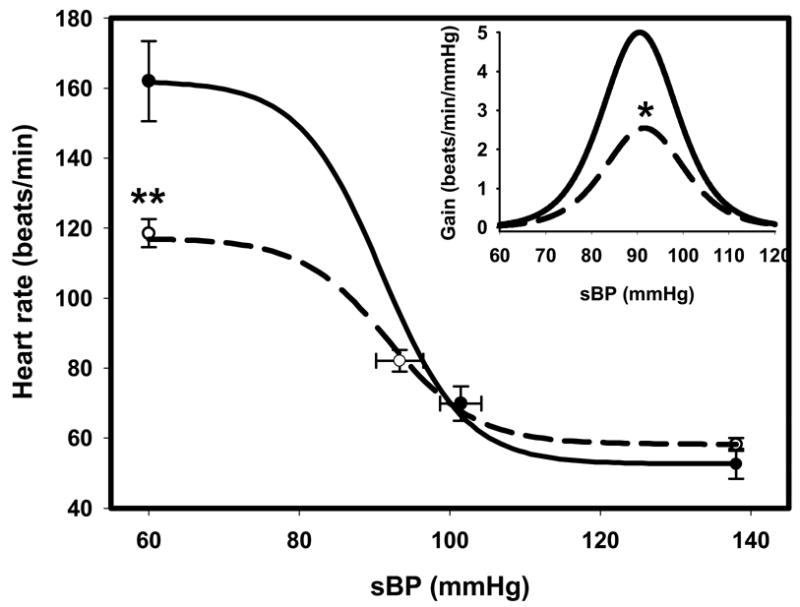
Composite baroreflex relations between heart rate (HR) and systolic blood pressure (sBP) from normal sheep (solid line; n=8) and sheep in heart failure (HF, dashed line; n=8). *P<0.05, **P<0.01 compared with normal group.
Table 2.
Values for maximum gains and top plateau and bottom plateau from normal animals and animals in heart failure (n=8 both groups).
| Normal | Heart Failure | |
|---|---|---|
| sBP vs HR | ||
| Maximum gain (beats/min/mmHg) | −5.34 ± 0.66 | −3.29 ± 0.56 * |
| Top plateau (beats/min) | 162.0 ± 11.44 | 117.1 ± 4.3 *** |
| Bottom plateau (beats/min) | 52.6 ± 4.3 | 58.2 ± 1.8 |
| dBP vs normalized CSNA | ||
| Maximum gain (% max/mmHg) | −6.03 ± 0.95 | −6.33 ± 1.06 |
| dBP vs CSNA | ||
| Maximum gain (spikes/sec/mmHg) | −2.30 ± 0.41 | −5.51 ± 1.60 |
| Top plateau (spikes/sec) | 43.8 ± 9.9 | 76.8 ± 14.5 |
| Bottom plateau (spikes/sec) | −2.47 ± 3.5 | −3.0 ± 2.2 |
Abbreviations: CSNA, cardiac sympathetic nerve activity; dBP, diastolic blood pressure; HP, heart period; HR, heart rate.
P<0.05,
P<0.01,
P<0.001 vs normal group.
In contrast, the gains of the baroreflex relations between diastolic BP and normalized CSNA were not significantly different in the HF (−6.33 ± 1.06 % max/mmHg) compared with the normal group (−6.03 ± 0.95 % max/mmHg) (Figure 4, Table 2). The baroreflex relationship between diastolic BP and raw CSNA spike counts showed a non-significant tendency to an increased gain and upper plateau in HF sheep (Table 2).
Figure 4.
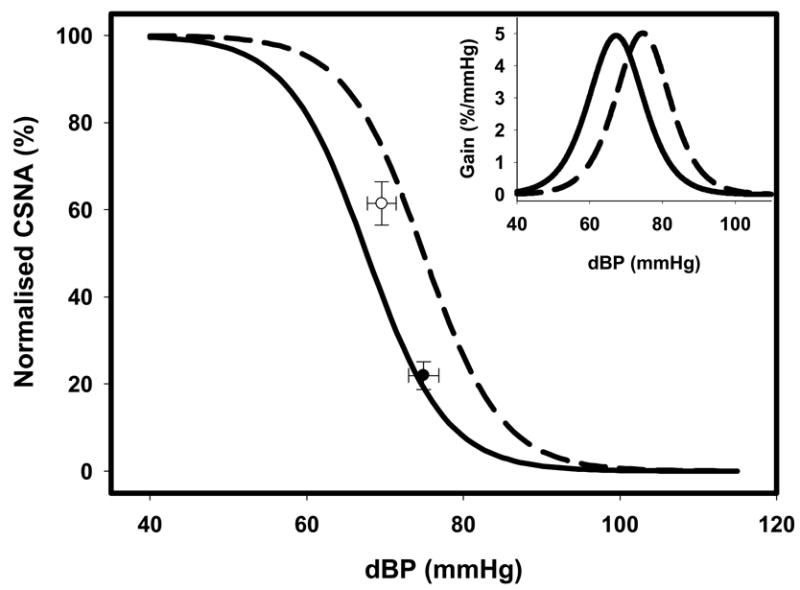
Composite baroreflex relations between normalised cardiac sympathetic nerve activity (CSNA) and diastolic blood pressure (dBP) in normal sheep (solid line; n=8) and sheep in heart failure (HF, dashed line; n=8).
Discussion
The unique feature of this study is the direct recording of CSNA in conscious animals with HF. Using this technique we have shown for the first time that in HF there is a striking increase in CSNA, as demonstrated by almost a doubling in burst frequency in conscious sheep with pacing-induced HF compared with normal sheep. Furthermore, in HF, resting CSNA was held at a level much closer to its upper plateau attained during baroreceptor unloading than was the case in normal sheep. As expected from observations in humans and other species (11, 14, 17), in HF there was a desensitization of the relation between HR and arterial pressure; but, crucially, there was no blunting of the arterial baroreflex control of CSNA.
The majority of direct information on the control of SNA in HF has been obtained from measurement of RSNA in animals and MSNA in humans, and in both cases large increases in activity have been found in HF (9, 14, 17, 18, 28). Due to the heterogeneity of control of sympathetic outflow, the changes in resting activity and baroreflex control of CSNA cannot be predicted from the measurement of other sympathetic outflows. For example, in the normal state the bursting pattern of CSNA is not identical to that of other sympathetic nerves (37), the time course of the response to behavioural stimuli differs (37), activation of left atrial receptors stimulates CSNA but decreases RSNA (21) and anesthesia has a greater depressant effect on CSNA compared with RSNA (32). Furthermore, we have demonstrated that central infusion of angiotensin II had opposite effects on CSNA and RSNA in conscious sheep (33, 47).
Although CSNA has not been recorded previously in HF, cardiac norepinephrine spillover has been measured in HF patients. Interestingly these studies demonstrated that cardiac norepinephrine spillover was increased by 10–50 fold in severe HF compared with increases of approximately 3 fold to the kidney and less to other organs (19). In addition, cardiac norepinephrine spillover was increased early in HF, when spillover from other organs was not increased (41). These data were interpreted as indicating that in HF there are mechanisms that cause a preferential increase in CSNA. Cardiac norepinephrine spillover is, however, influenced by norepinephrine uptake, with evidence that uptake is reduced or impaired in HF (12, 41). Thus, the extent to which the increase in cardiac norepinephrine spillover depends on an increase in CSNA remained uncertain.
Our direct measurements of resting CSNA in normal sheep showed a wide inter-individual range of burst rates (20 to 70 %), which may be compared with the wide range reported for resting MSNA in normal humans (10–60 bursts/min) (13). The present study shows that in animals in HF, with an ejection fraction of 35–40 %, the resting CSNA burst rate increases to around 90% of heart beats. This level of activity is greater than that usually reported for MSNA in humans with HF (burst rate 60–70% of heart beats (6, 14, 17, 25)), although similarly high burst rates have been reported for RSNA in rats after myocardial infarction (16).
The observation that CSNA retained its pulse-synchronous character in HF indicates that the arterial baroreflex was still functional. Testing the full range of responses to arterial pressure changes showed clearly that the control of CSNA by arterial baroreflexes was not only present in HF sheep, but was at least as powerful as in normal animals. While this conclusion contrasts with reports of desensitized baroreflex control of MSNA in HF patients (14, 17, 25) and of RSNA in experimental animals (9, 26), preserved arterial baroreflex control of these sympathetic nerves has been reported by others (6, 7, 48). Crucially, these observations tell us that while CSNA was strongly activated in this model of HF, the activation could not have been due to dysfunctional baroreflex control.
A consistent finding in studies of HF is that the baroreflex control of heart rate is desensitized (5, 11, 14). Numerous studies have shown that vagal tone and parasympathetic nervous control of the heart are depressed in HF (3, 10, 11, 40), and this is likely to be the major reason that baroreflex control of HR is blunted. Indeed our finding that heart rate variability was significantly decreased indicates that vagal tone was reduced in the paced sheep. Although it is possible that abnormal baroreflex control of CSNA could be a contributing factor to the blunted heart rate response to hypotension, the present findings do not support such a view. It is likely that factors such as the down-regulation of cardiac β-receptors in HF, uncoupling of cardiac β-receptors from second messenger systems (29) and reduced density of cardiac sympathetic noradrenaline neurons in HF (20) contribute to this blunted response. Previous studies using spectral analysis of heart rate variability have suggested that the low frequency power in HR is a good index of cardiac sympathetic tone in the normal state (30, 39). Our recordings of CSNA in normal and HF sheep allowed us to test directly whether the difference in CSNA between the two states is reflected in the low frequency component of HR variability. Our data showed clearly that it was not a useful measure of the changes in CSNA which occur in HF, confirming the conclusions from previous studies using MSNA (24). Interestingly, we observed a reduction in the low frequency spectral power in CSNA during HF (Figure 2). This is in accord with previous studies that have observed a reduction in the low frequency spectral power in muscle SNA in HF patients (1, 45).
Limitations
The sheep used in these studies had ejection fractions of 35–40%, so we cannot exclude the possibility that in more severe HF (e.g. with ejection fraction ~ 20%), recruitment of further mechanisms might lead to desensitisation of the baroreflex control of CSNA. What we can say, however, is that desensitization of the arterial baroreflex was not a causative factor in the large increases in CSNA seen at this stage of HF in our experiments. It is also evident that reflexes from arterial baroreceptors cannot be the sole drive to increase SNA in HF, because sino-aortic denervation has been found not to reduce the increased plasma norepinephrine in dogs with pacing-induced HF (4). Other mechanisms that may stimulate CSNA in HF include cardiac mechano- and chemo-reflexes (2, 22, 36, 42), increased circulating levels of hormones such as angiotensin II and endothelin (8, 27, 35), and altered central mechanisms that may act to amplify the effects of afferent inputs (8, 49). Further studies are required to determine the relative importance of these mechanisms as causes of the increased CSNA in HF.
In conclusion, we have found that in conscious sheep with HF induced by rapid ventricular pacing, directly recorded CSNA was greatly increased compared to normal healthy sheep. Although the baroreflex control of HR was desensitized in sheep with HF compared to that in normal animals, the baroreflex control of CSNA was not significantly changed. These data indicate that the increased CSNA in HF is not dependent on depressed arterial baroreflex control of CSNA.
Acknowledgments
The authors are grateful to Craig Thomson for his excellent technical assistance and to David Trevaks for Spike 2 programming.
Footnotes
Disclosures
None.
Grants
This work was supported by grants from the National Health and Medical Research Council of Australia (232313) and the National Institutes of Health, USA (5 R01 HL07 4932).
References
- 1.Ando S, Dajani HR, Floras JS. Frequency domain characteristics of muscle sympathetic nerve activity in heart failure and healthy humans. Am J Physiol. 1997;273:R205–212. doi: 10.1152/ajpregu.1997.273.1.R205. [DOI] [PubMed] [Google Scholar]
- 2.Azevedo ER, Newton GE, Floras JS, Parker JD. Reducing cardiac filling pressure lowers norepinephrine spillover in patients with chronic heart failure. Circulation. 2000;101:2053–2059. doi: 10.1161/01.cir.101.17.2053. [DOI] [PubMed] [Google Scholar]
- 3.Bibevski S, Dunlap ME. Ganglionic mechanisms contribute to diminished vagal control in heart failure. Circulation. 1999;99:2958–2963. doi: 10.1161/01.cir.99.22.2958. [DOI] [PubMed] [Google Scholar]
- 4.Brändle M, Patel KP, Wang W, Zucker IH. Hemodynamic and norepinephrine responses to pacing-induced heart failure in conscious sinoaortic-denervated dogs. J Appl Physiol. 1996;81:1855–1862. doi: 10.1152/jappl.1996.81.4.1855. [DOI] [PubMed] [Google Scholar]
- 5.Chen JS, Wang W, Cornish KG, Zucker IH. Baro- and ventricular reflexes in conscious dogs subjected to chronic tachycardia. Am J Physiol. 1992;263:H1084–1089. doi: 10.1152/ajpheart.1992.263.4.H1084. [DOI] [PubMed] [Google Scholar]
- 6.Dibner-Dunlap ME, Smith ML, Kinugawa T, Thames MD. Enalaprilat augments arterial and cardiopulmonary baroreflex control of sympathetic nerve activity in patients with heart failure. J Am Coll Cardiol. 1996;27:358–364. doi: 10.1016/0735-1097(95)00484-x. [DOI] [PubMed] [Google Scholar]
- 7.Dibner-Dunlap ME, Thames MD. Baroreflex control of renal sympathetic nerve activity is preserved in heart failure despite reduced arterial baroreceptor sensitivity. Circ Res. 1989;65:1526–1535. doi: 10.1161/01.res.65.6.1526. [DOI] [PubMed] [Google Scholar]
- 8.DiBona GF, Jones SY, Brooks VL. ANG II receptor blockade and arterial baroreflex regulation of renal nerve activity in cardiac failure. Am J Physiol. 1995;269:R1189–1196. doi: 10.1152/ajpregu.1995.269.5.R1189. [DOI] [PubMed] [Google Scholar]
- 9.DiBona GF, Sawin LL. Reflex regulation of renal nerve activity in cardiac failure. Am J Physiol. 1994;266:R27–39. doi: 10.1152/ajpregu.1994.266.1.R27. [DOI] [PubMed] [Google Scholar]
- 10.Dunlap ME, Bibevski S, Rosenberry TL, Ernsberger P. Mechanisms of altered vagal control in heart failure: influence of muscarinic receptors and acetylcholinesterase activity. Am J Physiol Heart Circ Physiol. 2003;285:H1632–1640. doi: 10.1152/ajpheart.01051.2002. [DOI] [PubMed] [Google Scholar]
- 11.Eckberg DL, Drabinsky M, Braunwald E. Defective cardiac parasympathetic control in patients with heart disease. N Engl J Med. 1971;285:877–883. doi: 10.1056/NEJM197110142851602. [DOI] [PubMed] [Google Scholar]
- 12.Eisenhofer G, Friberg P, Rundqvist B, Quyyumi AA, Lambert G, Kaye DM, Kopin IJ, Goldstein DS, Esler MD. Cardiac sympathetic nerve function in congestive heart failure. Circulation. 1996;93:1667–1676. doi: 10.1161/01.cir.93.9.1667. [DOI] [PubMed] [Google Scholar]
- 13.Fagius J, Wallin BG. Long-term variability and reproducibility of resting human muscle nerve sympathetic activity at rest, as reassessed after a decade. Clin Auton Res. 1993;3:201–205. doi: 10.1007/BF01826234. [DOI] [PubMed] [Google Scholar]
- 14.Ferguson DW, Berg WJ, Roach PJ, Oren RM, Mark AL. Effects of heart failure on baroreflex control of sympathetic neural activity. Am J Cardiol. 1992;69:523–531. doi: 10.1016/0002-9149(92)90998-e. [DOI] [PubMed] [Google Scholar]
- 15.Floras JS. Arterial baroreceptor and cardiopulmonary reflex control of sympathetic outflow in human heart failure. Ann N Y Acad Sci. 2001;940:500–513. doi: 10.1111/j.1749-6632.2001.tb03701.x. [DOI] [PubMed] [Google Scholar]
- 16.Francis J, Weiss RM, Wei SG, Johnson AK, Beltz TG, Zimmerman K, Felder RB. Central mineralocorticoid receptor blockade improves volume regulation and reduces sympathetic drive in heart failure. Am J Physiol Heart Circ Physiol. 2001;281:H2241–2251. doi: 10.1152/ajpheart.2001.281.5.H2241. [DOI] [PubMed] [Google Scholar]
- 17.Grassi G, Seravalle G, Cattaneo BM, Lanfranchi A, Vailati S, Giannattasio C, Del Bo A, Sala C, Bolla GB, Pozzi M. Sympathetic activation and loss of reflex sympathetic control in mild congestive heart failure. Circulation. 1995;92:3206–3211. doi: 10.1161/01.cir.92.11.3206. [DOI] [PubMed] [Google Scholar]
- 18.Hara K, Floras JS. After-effects of exercise on haemodynamics and muscle sympathetic nerve activity in young patients with dilated cardiomyopathy. Heart. 1996;75:602–608. doi: 10.1136/hrt.75.6.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hasking GJ, Esler MD, Jennings GL, Burton D, Johns JA, Korner PI. Norepinephrine spillover to plasma in patients with congestive heart failure: evidence of increased overall and cardiorenal sympathetic nervous activity. Circulation. 1986;73:615–621. doi: 10.1161/01.cir.73.4.615. [DOI] [PubMed] [Google Scholar]
- 20.Himura Y, Felten SY, Kashiki M, Lewandowski TJ, Delehanty JM, Liang CS. Cardiac noradrenergic nerve terminal abnormalities in dogs with experimental congestive heart failure. Circulation. 1993;88:1299–1309. doi: 10.1161/01.cir.88.3.1299. [DOI] [PubMed] [Google Scholar]
- 21.Karim F, Kidd C, Malpus CM, Penna PE. The effects of stimulation of the left atrial receptors on sympathetic efferent nerve activity. J Physiol. 1972;227:243–260. doi: 10.1113/jphysiol.1972.sp010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaye DM, Lambert GW, Lefkovits J, Morris M, Jennings G, Esler MD. Neurochemical evidence of cardiac sympathetic activation and increased central nervous system norepinephrine turnover in severe congestive heart failure. J Am Coll Cardiol. 1994;23:570–578. doi: 10.1016/0735-1097(94)90738-2. [DOI] [PubMed] [Google Scholar]
- 23.Kaye DM, Lefkovits J, Jennings GL, Bergin P, Broughton A, Esler MD. Adverse consequences of high sympathetic nervous activity in the failing human heart. J Am Coll Cardiol. 1995;26:1257–1263. doi: 10.1016/0735-1097(95)00332-0. [DOI] [PubMed] [Google Scholar]
- 24.Kingwell BA, Thompson JM, Kaye DM, McPherson GA, Jennings GL, Esler MD. Heart rate spectral analysis, cardiac norepinephrine spillover, and muscle sympathetic nerve activity during human sympathetic nervous activation and failure. Circulation. 1994;90:234–240. doi: 10.1161/01.cir.90.1.234. [DOI] [PubMed] [Google Scholar]
- 25.Leimbach WN, Jr, Wallin BG, Victor RG, Aylward PE, Sundlof G, Mark AL. Direct evidence from intraneural recordings for increased central sympathetic outflow in patients with heart failure. Circulation. 1986;73:913–919. doi: 10.1161/01.cir.73.5.913. [DOI] [PubMed] [Google Scholar]
- 26.Liu JL, Irvine S, Reid IA, Patel KP, Zucker IH. Chronic exercise reduces sympathetic nerve activity in rabbits with pacing-induced heart failure: A role for angiotensin II. Circulation. 2000;102:1854–1862. doi: 10.1161/01.cir.102.15.1854. [DOI] [PubMed] [Google Scholar]
- 27.Liu JL, Pliquett RU, Brewer E, Cornish KG, Shen YT, Zucker IH. Chronic endothelin-1 blockade reduces sympathetic nerve activity in rabbits with heart failure. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1906–1913. doi: 10.1152/ajpregu.2001.280.6.R1906. [DOI] [PubMed] [Google Scholar]
- 28.Liu JL, Zucker IH. Regulation of sympathetic nerve activity in heart failure: a role for nitric oxide and angiotensin II. Circ Res. 1999;84:417–423. doi: 10.1161/01.res.84.4.417. [DOI] [PubMed] [Google Scholar]
- 29.Lohse MJ, Engelhardt S, Eschenhagen T. What is the role of beta-adrenergic signaling in heart failure? Circ Res. 2003;93:896–906. doi: 10.1161/01.RES.0000102042.83024.CA. [DOI] [PubMed] [Google Scholar]
- 30.Malliani A, Pagani M, Lombardi F, Cerutti S. Cardiovascular neural regulation explored in the frequency domain. Circulation. 1991;84:482–492. doi: 10.1161/01.cir.84.2.482. [DOI] [PubMed] [Google Scholar]
- 31.Mark AL. Sympathetic dysregulation in heart failure: mechanisms and therapy. Clin Cardiol. 1995;18:I3–8. doi: 10.1002/clc.4960181303. [DOI] [PubMed] [Google Scholar]
- 32.Matsukawa K, Ninomiya I, Nishiura N. Effects of anesthesia on cardiac and renal sympathetic nerve activities and plasma catecholamines. Am J Physiol. 1993;265:R792–797. doi: 10.1152/ajpregu.1993.265.4.R792. [DOI] [PubMed] [Google Scholar]
- 33.May CN, McAllen RM. Baroreceptor-independent renal nerve inhibition by intracerebroventricular angiotensin II in conscious sheep. Am J Physiol. 1997;273:R560–567. doi: 10.1152/ajpregu.1997.273.2.R560. [DOI] [PubMed] [Google Scholar]
- 34.McKibben JS, Getty R. A comparative sudy of the cardiac innervation in domestic animals: sheep. Acta anat. 1969;74:228–242. doi: 10.1159/000143379. [DOI] [PubMed] [Google Scholar]
- 35.Murakami H, Liu JL, Zucker IH. Blockade of AT1 receptors enhances baroreflex control of heart rate in conscious rabbits with heart failure. Am J Physiol. 1996;271:R303–309. doi: 10.1152/ajpregu.1996.271.1.R303. [DOI] [PubMed] [Google Scholar]
- 36.Narkiewicz K, Pesek CA, van de Borne PJ, Kato M, Somers VK. Enhanced sympathetic and ventilatory responses to central chemoreflex activation in heart failure. Circulation. 1999;100:262–267. doi: 10.1161/01.cir.100.3.262. [DOI] [PubMed] [Google Scholar]
- 37.Ninomiya I, Matsukawa K, Nishiura N. Central and baroreflex control of sympathetic nerve activity to the heart and kidney in a daily life of the cat. Clinical and and Experimental- Theory and Practise. 1988;A10(Suppl 1):19–31. doi: 10.3109/10641968809075961. [DOI] [PubMed] [Google Scholar]
- 38.Packer M, Coats AJ, Fowler MB, Katus HA, Krum H, Mohacsi P, Rouleau JL, Tendera M, Castaigne A, Roecker EB, Schultz MK, DeMets DL. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med. 2001;344:1651–1658. doi: 10.1056/NEJM200105313442201. [DOI] [PubMed] [Google Scholar]
- 39.Pagani M, Lombardi F, Guzzetti S, Rimoldi O, Furlan R, Pizzinelli P, Sandrone G, Malfatto G, Dell'Orto S, Piccaluga E, et al. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ Res. 1986;59:178–193. doi: 10.1161/01.res.59.2.178. [DOI] [PubMed] [Google Scholar]
- 40.Porter TR, Eckberg DL, Fritsch JM, Rea RF, Beightol LA, Schmedtje JF, Jr, Mohanty PK. Autonomic pathophysiology in heart failure patients. Sympathetic-cholinergic interrelations. J Clin Invest. 1990;85:1362–1371. doi: 10.1172/JCI114580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rundqvist B, Elam M, Bergmann-Sverrisdottir Y, Eisenhofer G, Friberg P. Increased cardiac adrenergic drive precedes generalized sympathetic activation in human heart failure. Circulation. 1997;95:169–175. doi: 10.1161/01.cir.95.1.169. [DOI] [PubMed] [Google Scholar]
- 42.Sun SY, Wang W, Zucker IH, Schultz HD. Enhanced peripheral chemoreflex function in conscious rabbits with pacing-induced heart failure. J Appl Physiol. 1999;86:1264–1272. doi: 10.1152/jappl.1999.86.4.1264. [DOI] [PubMed] [Google Scholar]
- 43.Sundlöf G, Wallin BG. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. J Physiol. 1978;274:621–637. doi: 10.1113/jphysiol.1978.sp012170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thomas JA, Marks BH. Plasma norepinephrine in congestive heart failure. Am J Cardiol. 1978;41:233–243. doi: 10.1016/0002-9149(78)90162-5. [DOI] [PubMed] [Google Scholar]
- 45.van de Borne P, Montano N, Pagani M, Oren R, Somers VK. Absence of low-frequency variability of sympathetic nerve activity in severe heart failure. Circulation. 1997;95:1449–1454. doi: 10.1161/01.cir.95.6.1449. [DOI] [PubMed] [Google Scholar]
- 46.Waites GM. The course of the efferent cardiac nerves of the sheep. J Physiol. 1957;139:417–433. doi: 10.1113/jphysiol.1957.sp005902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watson AMD, Mogulkoc R, McAllen RM, May CN. Stimulation of cardiac sympathetic nerve activity by central angiotensinergic mechanisms in conscious sheep. Am J Physiol Regul Integr Comp Physiol. 2004;286:R1051–1056. doi: 10.1152/ajpregu.00708.2003. [DOI] [PubMed] [Google Scholar]
- 48.Zucker IH, Gorman AJ, Cornish KG, Lang M. Imparied atrial receptor modulation or renal nerve activity in dogs with chronic volume overload. Cardiovasc Res. 1985;19:411–418. doi: 10.1093/cvr/19.7.411. [DOI] [PubMed] [Google Scholar]
- 49.Zucker IH, Schultz HD, Li YF, Wang Y, Wang W, Patel KP. The origin of sympathetic outflow in heart failure: the roles of angiotensin II and nitric oxide. Prog Biophys Mol Biol. 2004;84:217–232. doi: 10.1016/j.pbiomolbio.2003.11.010. [DOI] [PubMed] [Google Scholar]


