Abstract
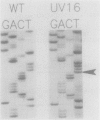
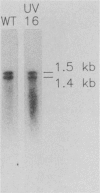
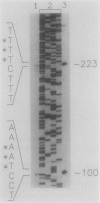
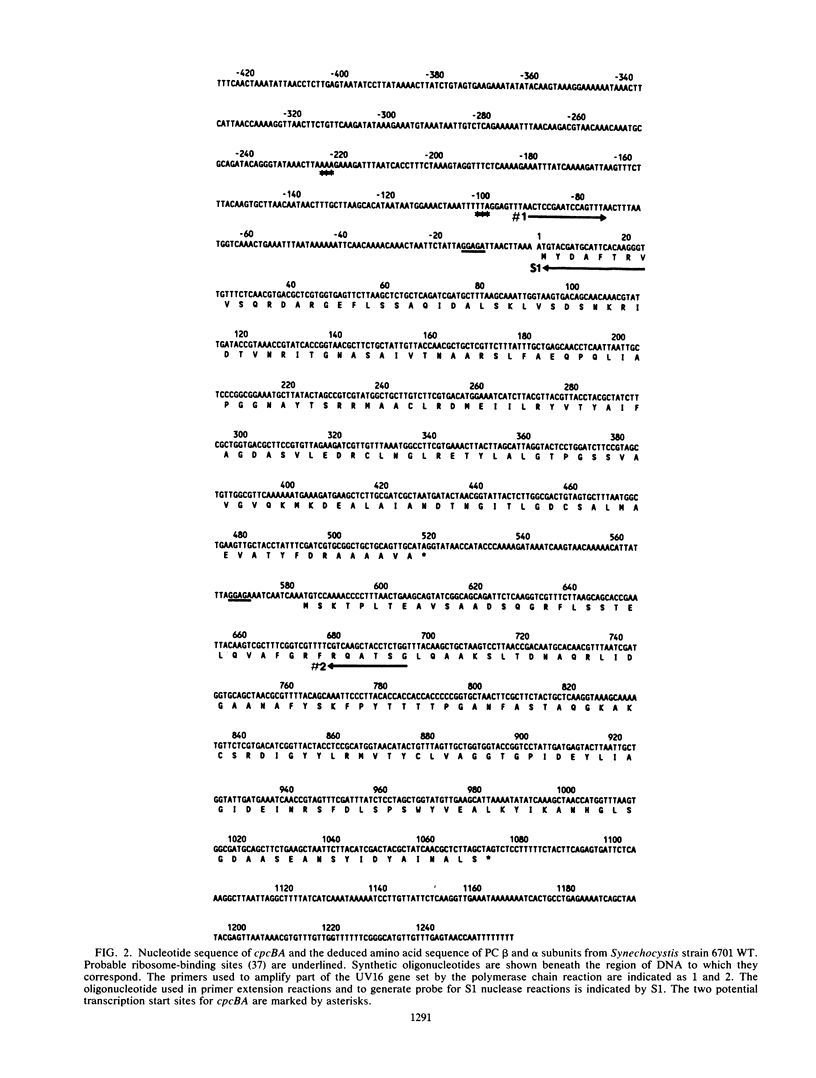
The cyanobacterial phycobilisome is a large protein complex located on the photosynthetic membrane. It harvests light energy and transfers it to chlorophyll for use in photosynthesis. Phycobilisome assembly mutants in the unicellular cyanobacterium Synechocystis sp. strain 6701 have been characterized. One such mutant, UV16, contains a defect in the assembly of the biliprotein phycocyanin. We report the cloning and sequencing of the phycocyanin genes from wild-type Synechocystis strain 6701 and demonstrate an alteration in the gene for the phycocyanin alpha subunit in UV16. Possible consequences of the lesion on phycobilisome assembly were assessed from its position in the phycocyanin tertiary and quaternary structures. The UV16 phenotype is complex and includes a reduced level of phycocyanin relative to that in the wild type. To determine whether the lower phycocyanin content results from lower transcript levels, a fragment of cpcBA was used as a probe for quantitating phycocyanin mRNA. Both the wild type and UV16 contained two phycocyanin transcripts of approximately 1.4 and 1.5 kilobases that were equal in abundance and that did not vary with light quality during cell growth. Equal levels of these transcripts in the wild type and UV16 suggest that the lower phycocyanin content in the mutant may be due to posttranscriptional events. The 5' ends of the two phycocyanin mRNAs were mapped at 100 and 223 base pairs upstream of the cpcB initiation codon. Homologous regions upstream of the putative transcription initiation sites may be important for maintaining high levels of transcription from the Synechocystis strain 6701 phycocyanin gene set.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen M. M., Smith A. J. Nitrogen chlorosis in blue-green algae. Arch Mikrobiol. 1969;69(2):114–120. doi: 10.1007/BF00409755. [DOI] [PubMed] [Google Scholar]
- Anderson L. K., Eiserling F. A. Asymmetrical core structure in phycobilisomes of the cyanobacterium Synechocystis 6701. J Mol Biol. 1986 Oct 5;191(3):441–451. doi: 10.1016/0022-2836(86)90139-7. [DOI] [PubMed] [Google Scholar]
- Anderson L. K., Grossman A. R. Structure and light-regulated expression of phycoerythrin genes in wild-type and phycobilisome assembly mutants of Synechocystis sp. strain PCC 6701. J Bacteriol. 1990 Mar;172(3):1297–1305. doi: 10.1128/jb.172.3.1297-1305.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson L. K., Rayner M. C., Eiserling F. A. Mutations that affect structure and assembly of light-harvesting proteins in the cyanobacterium Synechocystis sp. strain 6701. J Bacteriol. 1987 Jan;169(1):102–109. doi: 10.1128/jb.169.1.102-109.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belknap W. R., Haselkorn R. Cloning and light regulation of expression of the phycocyanin operon of the cyanobacterium Anabaena. EMBO J. 1987 Apr;6(4):871–884. doi: 10.1002/j.1460-2075.1987.tb04833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellemare G., Bartlett S. G., Chua N. H. Biosynthesis of chlorophyll a/b-binding polypeptides in wild type and the chlorina f2 mutant of barley. J Biol Chem. 1982 Jul 10;257(13):7762–7767. [PubMed] [Google Scholar]
- Bruns B. U., Briggs W. R., Grossman A. R. Molecular characterization of phycobilisome regulatory mutants of Fremyella diplosiphon. J Bacteriol. 1989 Feb;171(2):901–908. doi: 10.1128/jb.171.2.901-908.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobley J. G., Miranda R. D. Mutations affecting chromatic adaptation in the cyanobacterium Fremyella diplosiphon. J Bacteriol. 1983 Mar;153(3):1486–1492. doi: 10.1128/jb.153.3.1486-1492.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley P. B., Lemaux P. G., Grossman A. R. Cyanobacterial light-harvesting complex subunits encoded in two red light-induced transcripts. Science. 1985 Nov 1;230(4725):550–553. doi: 10.1126/science.3931221. [DOI] [PubMed] [Google Scholar]
- Conley P. B., Lemaux P. G., Grossman A. Molecular characterization and evolution of sequences encoding light-harvesting components in the chromatically adapting cyanobacterium Fremyella diplosiphon. J Mol Biol. 1988 Feb 5;199(3):447–465. doi: 10.1016/0022-2836(88)90617-1. [DOI] [PubMed] [Google Scholar]
- Conley P. B., Lemaux P. G., Lomax T. L., Grossman A. R. Genes encoding major light-harvesting polypeptides are clustered on the genome of the cyanobacterium Fremyella diplosiphon. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3924–3928. doi: 10.1073/pnas.83.11.3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis S. E. Genes encoding the beta and epsilon subunits of the proton-translocating ATPase from Anabaena sp. strain PCC 7120. J Bacteriol. 1987 Jan;169(1):80–86. doi: 10.1128/jb.169.1.80-86.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Füglistaller P., Suter F., Zuber H. The complete amino-acid sequence of both subunits of phycoerythrocyanin from the thermophilic cyanobacterium Mastigocladus laminosus. Hoppe Seylers Z Physiol Chem. 1983 Jun;364(6):691–712. doi: 10.1515/bchm2.1983.364.1.691. [DOI] [PubMed] [Google Scholar]
- Glazer A. N., Lundell D. J., Yamanaka G., Williams R. C. The structure of a "simple" phycobilisome. Ann Microbiol (Paris) 1983 Jul-Aug;134B(1):159–180. doi: 10.1016/s0769-2609(83)80103-3. [DOI] [PubMed] [Google Scholar]
- Kalla R., Lind L. K., Gustafsson P. Genetic analysis of phycobilisome mutants in the cyanobacterium Synechococcus species PCC 6301. Mol Microbiol. 1989 Mar;3(3):339–347. doi: 10.1111/j.1365-2958.1989.tb00179.x. [DOI] [PubMed] [Google Scholar]
- Klotz A. V., Glazer A. N. gamma-N-methylasparagine in phycobiliproteins. Occurrence, location, and biosynthesis. J Biol Chem. 1987 Dec 25;262(36):17350–17355. [PubMed] [Google Scholar]
- Lemaux P. G., Grossman A. R. Major light-harvesting polypeptides encoded in polycistronic transcripts in a eukaryotic alga. EMBO J. 1985 Aug;4(8):1911–1919. doi: 10.1002/j.1460-2075.1985.tb03870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax T. L., Conley P. B., Schilling J., Grossman A. R. Isolation and characterization of light-regulated phycobilisome linker polypeptide genes and their transcription as a polycistronic mRNA. J Bacteriol. 1987 Jun;169(6):2675–2684. doi: 10.1128/jb.169.6.2675-2684.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundell D. J., Glazer A. N. Molecular architecture of a light-harvesting antenna. Core substructure in Synechococcus 6301 phycobilisomes: two new allophycocyanin and allophycocyanin B complexes. J Biol Chem. 1983 Jan 25;258(2):902–908. [PubMed] [Google Scholar]
- Lundell D. J., Glazer A. N. Molecular architecture of a light-harvesting antenna. Quaternary interactions in the Synechococcus 6301 phycobilisome core as revealed by partial tryptic digestion and circular dichroism studies. J Biol Chem. 1983 Jul 25;258(14):8708–8713. [PubMed] [Google Scholar]
- Lundell D. J., Glazer A. N. Molecular architecture of a light-harvesting antenna. Structure of the 18 S core-rod subassembly of the Synechococcus 6301 phycobilisome. J Biol Chem. 1983 Jan 25;258(2):894–901. [PubMed] [Google Scholar]
- Lundell D. J., Williams R. C., Glazer A. N. Molecular architecture of a light-harvesting antenna. In vitro assembly of the rod substructures of Synechococcus 6301 phycobilisomes. J Biol Chem. 1981 Apr 10;256(7):3580–3592. [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Offner G. D., Brown-Mason A. S., Ehrhardt M. M., Troxler R. F. Primary structure of phycocyanin from the unicellular rhodophyte Cyanidium caldarium. I. Complete amino acid sequence of the alpha subunit. J Biol Chem. 1981 Dec 10;256(23):12167–12175. [PubMed] [Google Scholar]
- Pilot T. J., Fox J. L. Cloning and sequencing of the genes encoding the alpha and beta subunits of C-phycocyanin from the cyanobacterium Agmenellum quadruplicatum. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6983–6987. doi: 10.1073/pnas.81.22.6983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riethman H. C., Mawhinney T. P., Sherman L. A. Phycobilisome-associated glycoproteins in the cyanobacterium Anacystis nidulans R 2. FEBS Lett. 1987 May 11;215(2):209–214. doi: 10.1016/0014-5793(87)80147-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmer T., Bode W., Huber R. Refined three-dimensional structures of two cyanobacterial C-phycocyanins at 2.1 and 2.5 A resolution. A common principle of phycobilin-protein interaction. J Mol Biol. 1987 Aug 5;196(3):677–695. doi: 10.1016/0022-2836(87)90040-4. [DOI] [PubMed] [Google Scholar]
- Schirmer T., Bode W., Huber R., Sidler W., Zuber H. X-ray crystallographic structure of the light-harvesting biliprotein C-phycocyanin from the thermophilic cyanobacterium Mastigocladus laminosus and its resemblance to globin structures. J Mol Biol. 1985 Jul 20;184(2):257–277. doi: 10.1016/0022-2836(85)90379-1. [DOI] [PubMed] [Google Scholar]
- Schirmer T., Huber R., Schneider M., Bode W., Miller M., Hackert M. L. Crystal structure analysis and refinement at 2.5 A of hexameric C-phycocyanin from the cyanobacterium Agmenellum quadruplicatum. The molecular model and its implications for light-harvesting. J Mol Biol. 1986 Apr 20;188(4):651–676. doi: 10.1016/s0022-2836(86)80013-4. [DOI] [PubMed] [Google Scholar]
- Schmidt G. W., Mishkind M. L. Rapid degradation of unassembled ribulose 1,5-bisphosphate carboxylase small subunits in chloroplasts. Proc Natl Acad Sci U S A. 1983 May;80(9):2632–2636. doi: 10.1073/pnas.80.9.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandeau de Marsac N. Occurrence and nature of chromatic adaptation in cyanobacteria. J Bacteriol. 1977 Apr;130(1):82–91. doi: 10.1128/jb.130.1.82-91.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- de Lorimier R., Bryant D. A., Porter R. D., Liu W. Y., Jay E., Stevens S. E., Jr Genes for the alpha and beta subunits of phycocyanin. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7946–7950. doi: 10.1073/pnas.81.24.7946. [DOI] [PMC free article] [PubMed] [Google Scholar]