Abstract
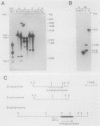
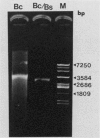
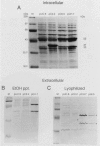
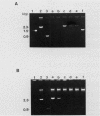
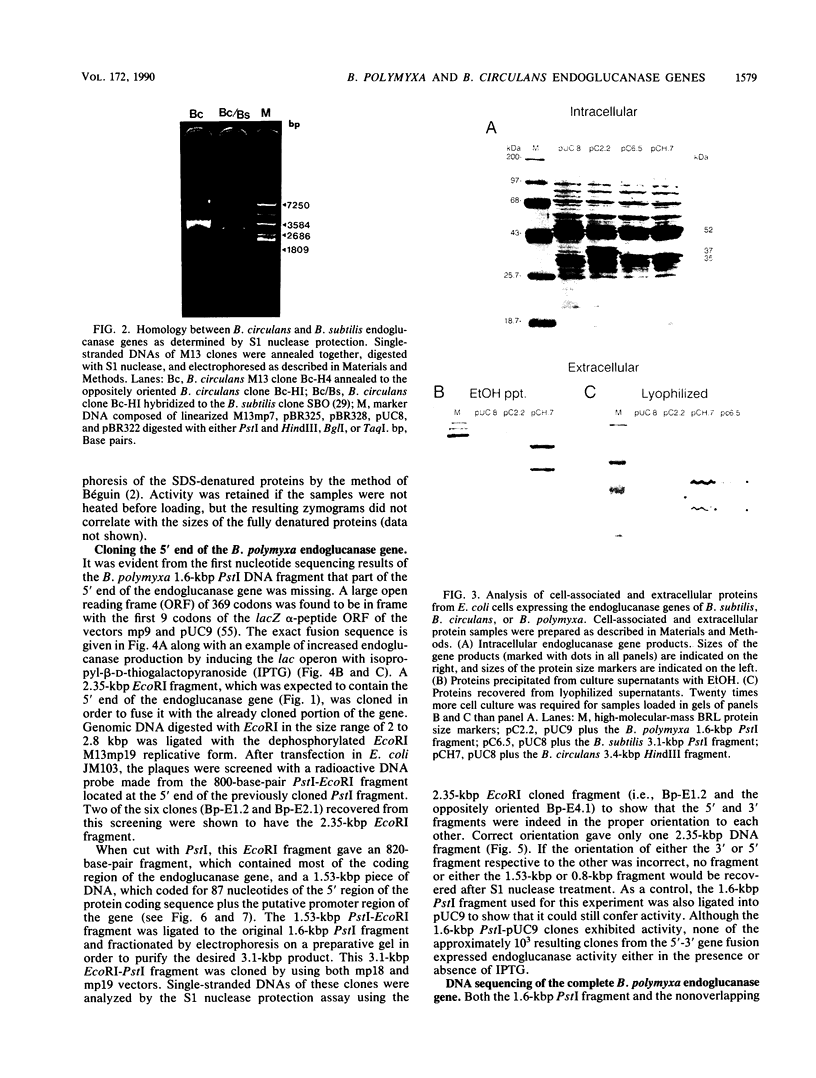
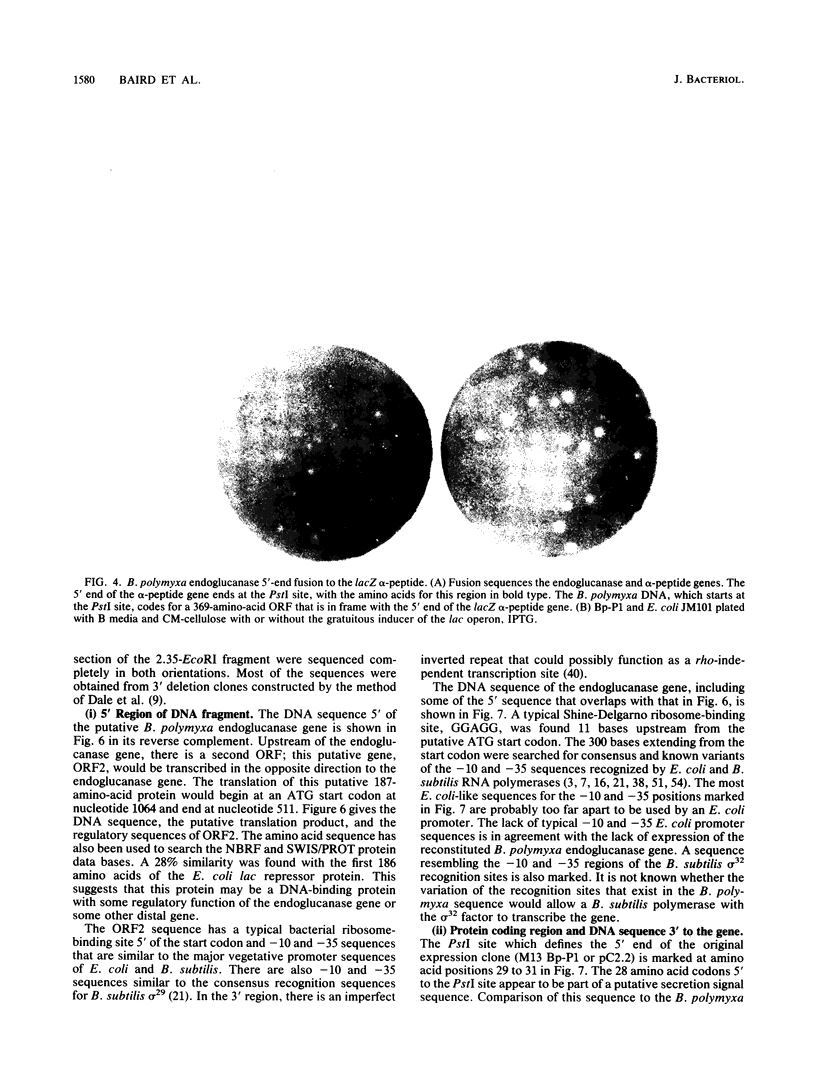
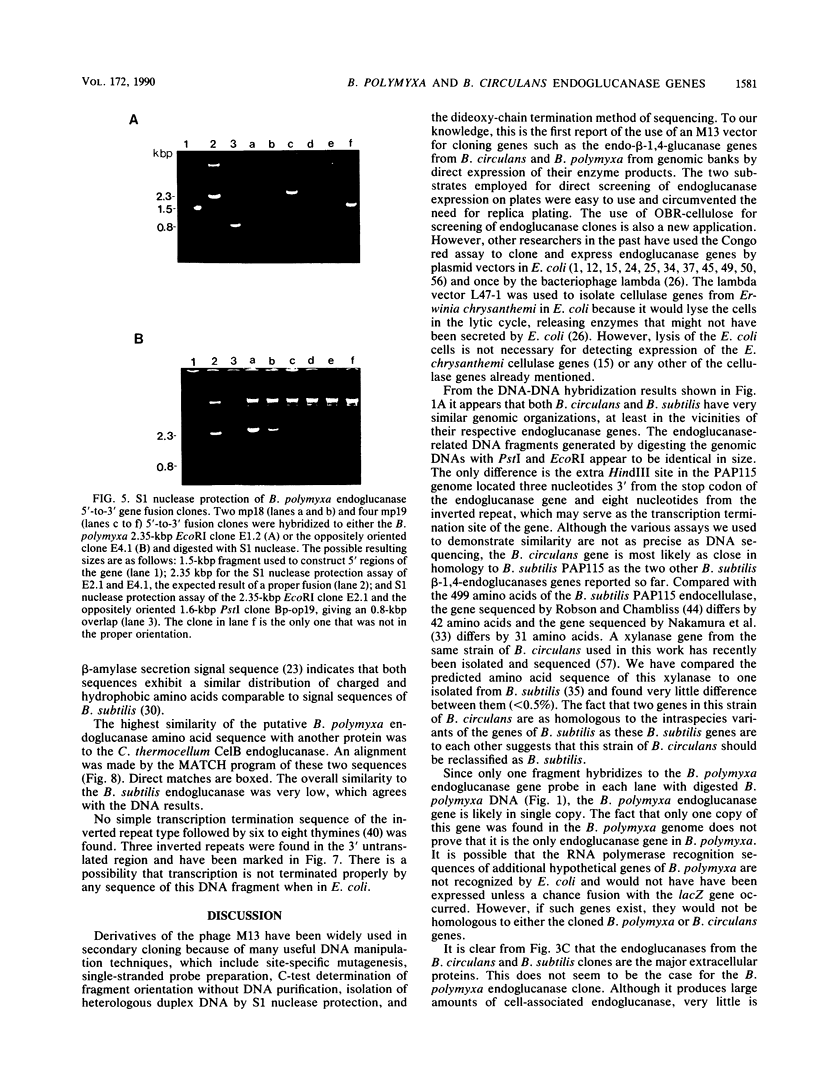
Endo-beta-1,4-glucanase genes from Bacillus circulans and from B. polymyxa were cloned by direct expression by using bacteriophage M13mp9 as the vector. The enzymatic activity of the gene products was detected by using either the Congo red assay or hydroxyethyl cellulose dyed with Ostazin Brilliant Red H-3B. The B. circulans and B. subtilis PAP115 endo-beta-1,4-glucanase genes were shown to be homologous by the use of restriction endonuclease site mapping, DNA-DNA hybridization, S1 nuclease digestion after heteroduplex formation, and sodium dodecyl sulfate-polyacrylamide gel electrophoresis of the protein products. Analysis of the nucleotide sequence of 3.1 kilobase pairs of cloned B. polymyxa DNA revealed two convergently transcribed open reading frames (ORFs) consisting of 398 codons (endoglucanase) and 187 codons (ORF2) and separated by 374 nucleotides. The coding region of the B. polymyxa endoglucanase gene would theoretically produce a 44-kilodalton preprotein. Expression of the B. polymyxa endoglucanase in Escherichia coli was due to a fusion of the endoglucanase gene at codon 30 with codon 9 of the lacZ alpha-peptide gene. The B. polymyxa endoglucanase has 34% amino acid similarity to the Clostridium thermocellum celB endoglucanase sequence but very little similarity to endoglucanases from other Bacillus species. ORF2 has 28% amino acid similarity to the NH2-terminal half of the E. coli lac repressor protein, which is responsible for DNA binding.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barros M. E., Thomson J. A. Cloning and expression in Escherichia coli of a cellulase gene from Ruminococcus flavefaciens. J Bacteriol. 1987 Apr;169(4):1760–1762. doi: 10.1128/jb.169.4.1760-1762.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz J. L. Affinities of tight-binding lactose repressors for wild-type and pseudo-operators. J Mol Biol. 1987 Jun 5;195(3):495–504. doi: 10.1016/0022-2836(87)90178-1. [DOI] [PubMed] [Google Scholar]
- Biely P., Mislovicová D., Toman R. Soluble chromogenic substrates for the assay of endo-1,4-beta-xylanases and endo-1,4-beta-glucanases. Anal Biochem. 1985 Jan;144(1):142–146. doi: 10.1016/0003-2697(85)90095-8. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner M., Bujard H. Promoter recognition and promoter strength in the Escherichia coli system. EMBO J. 1987 Oct;6(10):3139–3144. doi: 10.1002/j.1460-2075.1987.tb02624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béguin P. Detection of cellulase activity in polyacrylamide gels using Congo red-stained agar replicas. Anal Biochem. 1983 Jun;131(2):333–336. doi: 10.1016/0003-2697(83)90178-1. [DOI] [PubMed] [Google Scholar]
- Béguin P., Rocancourt M., Chebrou M. C., Aubert J. P. Mapping of mRNA encoding endoglucanase A from Clostridium thermocellum. Mol Gen Genet. 1986 Feb;202(2):251–254. doi: 10.1007/BF00331645. [DOI] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Dretzen G., Bellard M., Sassone-Corsi P., Chambon P. A reliable method for the recovery of DNA fragments from agarose and acrylamide gels. Anal Biochem. 1981 Apr;112(2):295–298. doi: 10.1016/0003-2697(81)90296-7. [DOI] [PubMed] [Google Scholar]
- Faure E., Bagnara C., Belaich A., Belaich J. P. Cloning and expression of two cellulase genes of Clostridium cellulolyticum in Escherichia coli. Gene. 1988 May 15;65(1):51–58. doi: 10.1016/0378-1119(88)90416-7. [DOI] [PubMed] [Google Scholar]
- Fukumori F., Sashihara N., Kudo T., Horikoshi K. Nucleotide sequences of two cellulase genes from alkalophilic Bacillus sp. strain N-4 and their strong homology. J Bacteriol. 1986 Nov;168(2):479–485. doi: 10.1128/jb.168.2.479-485.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gijsegem F., Toussaint A., Schoonejans E. In vivo cloning of the pectate lyase and cellulase genes of Erwinia chrysanthemi. EMBO J. 1985 Mar;4(3):787–792. doi: 10.1002/j.1460-2075.1985.tb03698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg N. M., Warren R. A., Kilburn D. G., Miller R. C., Jr Regulation, initiation, and termination of the cenA and cex transcripts of Cellulomonas fimi. J Bacteriol. 1987 Feb;169(2):646–653. doi: 10.1128/jb.169.2.646-653.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grépinet O., Béguin P. Sequence of the cellulase gene of Clostridium thermocellum coding for endoglucanase B. Nucleic Acids Res. 1986 Feb 25;14(4):1791–1799. doi: 10.1093/nar/14.4.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hanna Z., Fregeau C., Préfontaine G., Brousseau R. Construction of a family of universal expression plasmid vectors. Gene. 1984 Oct;30(1-3):247–250. doi: 10.1016/0378-1119(84)90128-8. [DOI] [PubMed] [Google Scholar]
- Johnson W. C., Moran C. P., Jr, Losick R. Two RNA polymerase sigma factors from Bacillus subtilis discriminate between overlapping promoters for a developmentally regulated gene. Nature. 1983 Apr 28;302(5911):800–804. doi: 10.1038/302800a0. [DOI] [PubMed] [Google Scholar]
- Kanehisa M. I. Los Alamos sequence analysis package for nucleic acids and proteins. Nucleic Acids Res. 1982 Jan 11;10(1):183–196. doi: 10.1093/nar/10.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawazu T., Nakanishi Y., Uozumi N., Sasaki T., Yamagata H., Tsukagoshi N., Udaka S. Cloning and nucleotide sequence of the gene coding for enzymatically active fragments of the Bacillus polymyxa beta-amylase. J Bacteriol. 1987 Apr;169(4):1564–1570. doi: 10.1128/jb.169.4.1564-1570.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. M., Kong I. S., Yu J. H. Molecular cloning of an endoglucanase gene from an alkalophilic Bacillus sp. and its expression in Escherichia coli. Appl Environ Microbiol. 1987 Nov;53(11):2656–2659. doi: 10.1128/aem.53.11.2656-2659.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotoujansky A., Diolez A., Boccara M., Bertheau Y., Andro T., Coleno A. Molecular cloning of Erwinia chrysanthemi pectinase and cellulase structural genes. EMBO J. 1985 Mar;4(3):781–785. doi: 10.1002/j.1460-2075.1985.tb03697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lo A. C., MacKay R. M., Seligy V. L., Willick G. E. Bacillus subtilis beta-1,4-endoglucanase products from intact and truncated genes are secreted into the extracellular medium by Escherichia coli. Appl Environ Microbiol. 1988 Sep;54(9):2287–2292. doi: 10.1128/aem.54.9.2287-2292.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKay R. M., Lo A., Willick G., Zuker M., Baird S., Dove M., Moranelli F., Seligy V. Structure of a Bacillus subtilis endo-beta-1,4-glucanase gene. Nucleic Acids Res. 1986 Nov 25;14(22):9159–9170. doi: 10.1093/nar/14.22.9159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Nakamura A., Uozumi T., Beppu T. Nucleotide sequence of a cellulase gene of Bacillus subtilis. Eur J Biochem. 1987 Apr 15;164(2):317–320. doi: 10.1111/j.1432-1033.1987.tb11060.x. [DOI] [PubMed] [Google Scholar]
- Ohmiya K., Nagashima K., Kajino T., Goto E., Tsukada A., Shimizu S. Cloning of the cellulase gene from Ruminococcus albus and its expression in Escherichia coli. Appl Environ Microbiol. 1988 Jun;54(6):1511–1515. doi: 10.1128/aem.54.6.1511-1515.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petre J., Longin R., Millet J. Purification and properties of an endo-beta-1,4-glucanase from Clostridium thermocellum. Biochimie. 1981 Jul;63(7):629–639. doi: 10.1016/s0300-9084(81)80061-2. [DOI] [PubMed] [Google Scholar]
- Pustell J., Kafatos F. C. A convenient and adaptable package of DNA sequence analysis programs for microcomputers. Nucleic Acids Res. 1982 Jan 11;10(1):51–59. doi: 10.1093/nar/10.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pustell J., Kafatos F. C. A convenient and adaptable package of computer programs for DNA and protein sequence management, analysis and homology determination. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):643–655. doi: 10.1093/nar/12.1part2.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson L. M., Chambliss G. H. Endo-beta-1,4-glucanase gene of Bacillus subtilis DLG. J Bacteriol. 1987 May;169(5):2017–2025. doi: 10.1128/jb.169.5.2017-2025.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romaniec M. P., Clarke N. G., Hazlewood G. P. Molecular cloning of Clostridium thermocellum DNA and the expression of further novel endo-beta-1,4-glucanase genes in Escherichia coli. J Gen Microbiol. 1987 May;133(5):1297–1307. doi: 10.1099/00221287-133-5-1297. [DOI] [PubMed] [Google Scholar]
- SAITO H., MIURA K. I. PREPARATION OF TRANSFORMING DEOXYRIBONUCLEIC ACID BY PHENOL TREATMENT. Biochim Biophys Acta. 1963 Aug 20;72:619–629. [PubMed] [Google Scholar]
- Salerno A. J., Lampen J. O. Differential transcription of the bla regulatory region during induction of beta-lactamase in Bacillus licheniformis. FEBS Lett. 1988 Jan 18;227(1):61–65. doi: 10.1016/0014-5793(88)81414-5. [DOI] [PubMed] [Google Scholar]
- Sashihara N., Kudo T., Horikoshi K. Molecular cloning and expression of cellulase genes of alkalophilic Bacillus sp. strain N-4 in Escherichia coli. J Bacteriol. 1984 May;158(2):503–506. doi: 10.1128/jb.158.2.503-506.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenlist U., Simpson R. B., Gilbert W. E. coli RNA polymerase interacts homologously with two different promoters. Cell. 1980 Jun;20(2):269–281. doi: 10.1016/0092-8674(80)90613-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Teather R. M., Wood P. J. Use of Congo red-polysaccharide interactions in enumeration and characterization of cellulolytic bacteria from the bovine rumen. Appl Environ Microbiol. 1982 Apr;43(4):777–780. doi: 10.1128/aem.43.4.777-780.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers A. A. Structure and function of E. coli promoter DNA. CRC Crit Rev Biochem. 1987;22(3):181–219. doi: 10.3109/10409238709101483. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wolff B. R., Mudry T. A., Glick B. R., Pasternak J. J. Isolation of Endoglucanase Genes from Pseudomonas fluorescens subsp. cellulosa and a Pseudomonas sp. Appl Environ Microbiol. 1986 Jun;51(6):1367–1369. doi: 10.1128/aem.51.6.1367-1369.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R. C., MacKenzie C. R., Narang S. A. Nucleotide sequence of a Bacillus circulans xylanase gene. Nucleic Acids Res. 1988 Jul 25;16(14B):7187–7187. doi: 10.1093/nar/16.14.7187. [DOI] [PMC free article] [PubMed] [Google Scholar]