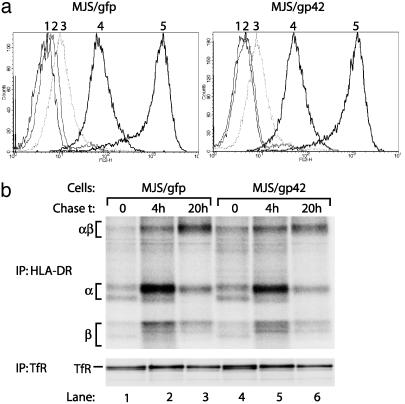
Fig. 3.
Surface expression and maturation of HLA-DR αβ-peptide complexes is not altered in MJS/gp42 cells. (a) Flow-cytometry histograms are depicted for surface staining of MJS/gfp and MJS/gp42 cells with the following mAbs: CerCLIP for HLA-DR/CLIP complexes (peak 2), Bü45 for Ii (peak 3), 16.23 for an HLA-DM-induced conformation of DR3 (peak 4), and L243 for HLA-DR (peak 5). As a negative control, no primary Ab was added (peak 1). (b) MJS/gp42 (lanes 4–6) and control MJS/gfp (lanes 1–3) cells were metabolically labeled for 1 h with [35S]methionine and chased for 0, 4, and 20 h, followed by cell lysis. HLA-DR complexes were isolated by immunoprecipitation (IP) with mAb L243, were incubated in nonreducing sample buffer with SDS at 37°C, which reveals the SDS-stable class II αβ dimers (αβ), and were analyzed on SDS/12% PAGE. As a control protein, TfR was precipitated by mAb 66IG10 and separated under reducing conditions by SDS/10% PAGE.