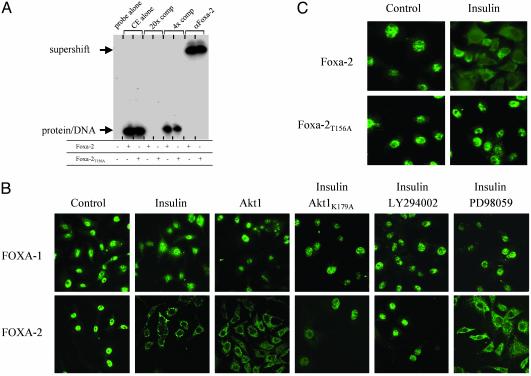
Fig. 5.
Phosphorylation of Foxa-2 by Akt-mediated insulin stimulation does not affect DNA binding but leads to nuclear exclusion of Foxa-2. (A) Cell extracts (CE) from insulin-stimulated HEK293 cells, transfected with Foxa-2 or Foxa-2T156A together with Akt (5 μg), were used in an electrophoretic mobility-shift assay. Foxa-2 binding site of Igfbp-1 (25) was used to shift proteins; a consensus Foxa-2 binding site was used for competition. Supershift was performed by using anti-Foxa-2 antibody. (B) Untransfected and Akt (125 ng)-transfected HepG2 cells treated with 50 nM insulin, LY294002, or 10 μM PD98059, alone or in combination, were decorated with anti-Foxa-1 or anti-Foxa-2 antibodies and visualized with an anti-rabbit IgG-Alexa 480 antibody by using laser scanning microscopy. (C) HepG2 cells were transfected with expression vectors for either HA-Foxa-2 or HA-Foxa-2T156A (100 ng) and treated with 50 nM insulin. Cells were decorated with an anti-HA-antibody and visualized with an anti-rabbit IgG-Alexa 480 antibody by using laser scanning microscopy. Control cells were starved for 10 h; all other experiments were performed in medium containing 10% FCS.