Abstract
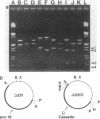
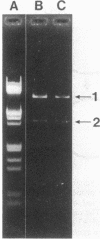
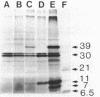
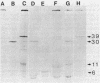
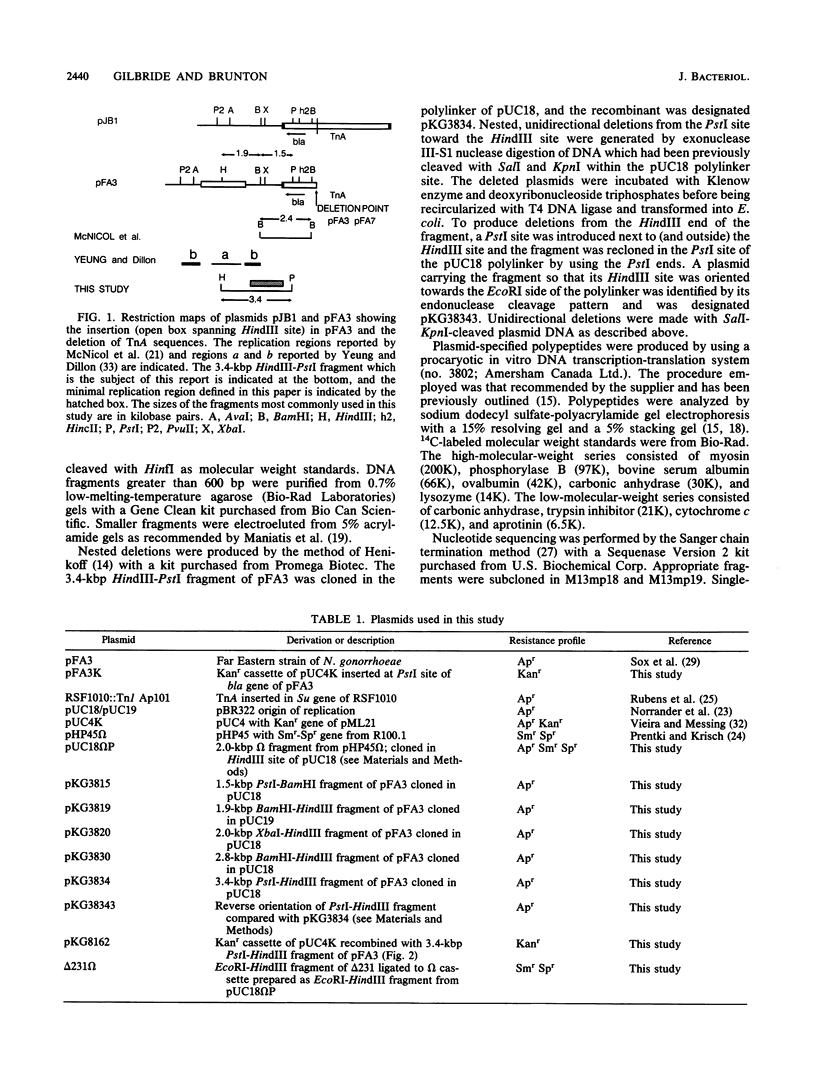
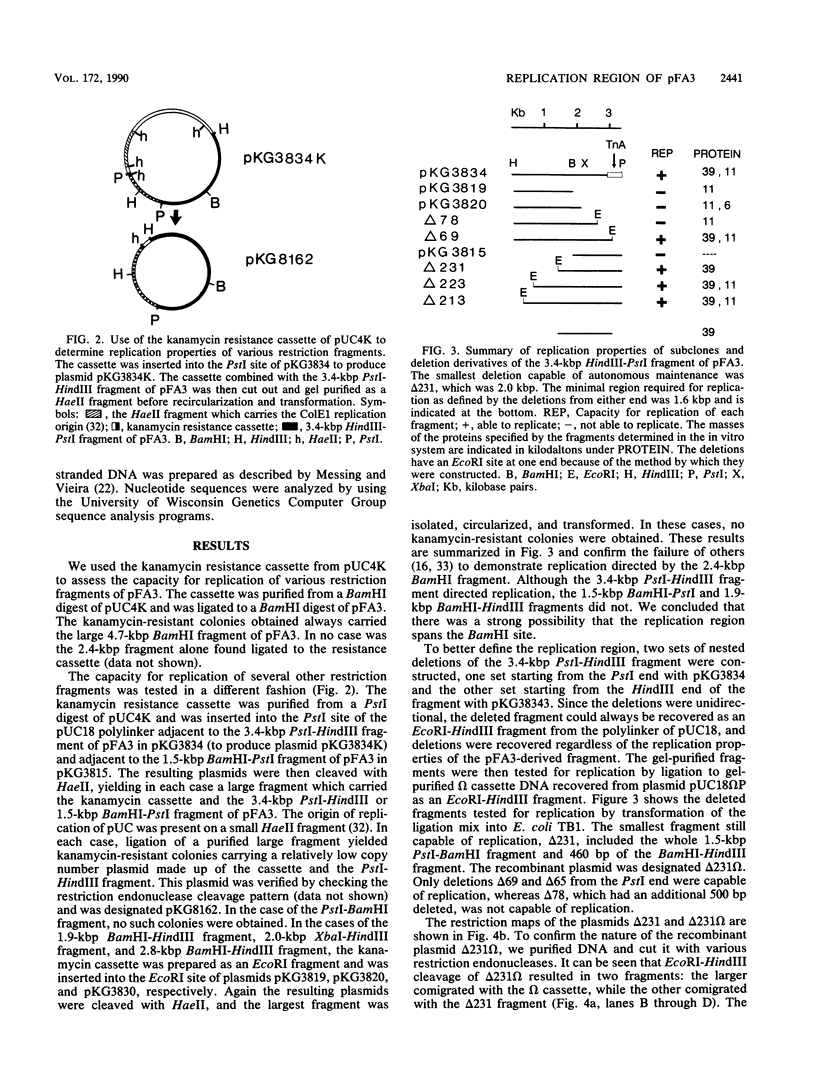
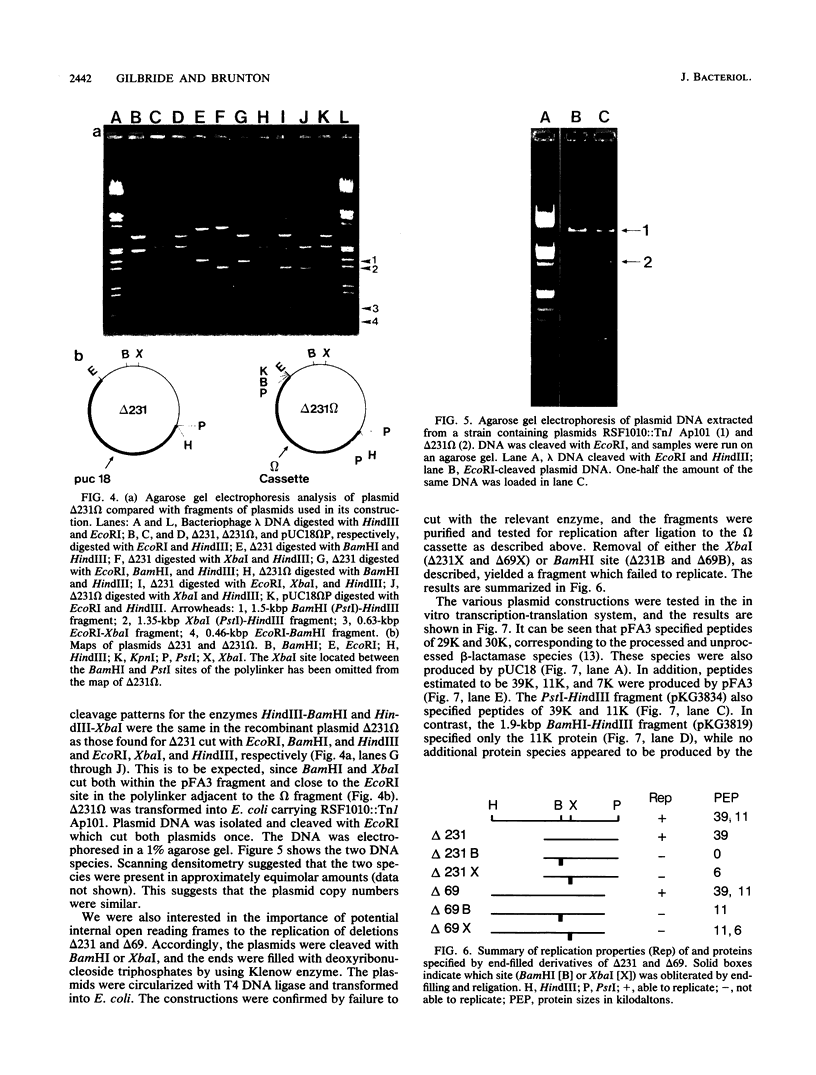
The 7.1-kilobase-pair (kbp) plasmid pFA3 specifies TEM beta-lactamase production in Neisseria gonorrhoeae. We studied the minimal region required for replication of this plasmid in Escherichia coli by constructing a set of nested deletions of the 3.4-kbp PstI-HindIII fragment. The smallest fragment capable of maintenance in E. coli when ligated to a streptomycin-spectinomycin resistance cassette was 2.0 kbp in size and was different from another autonomously replicating fragment of pFA3 reported by K. H. Yeung and J. Dillon (Plasmid 20:232-240, 1988). The fragment contained single BamHI and XbaI sites and specified a 39-K protein. Fragments subcloned from the minimal region or constructed by deletion from the 3' or 5' ends were not capable of autonomous replication. Mutants constructed by end filling and religating DNA cleaved at the BamHI or XbaI sites were not capable of autonomous replication and no longer produced the 39K protein. These results suggest that replication is dependent on the 39K protein. DNA sequence analysis of the region showed an A-T-rich region followed by four 22-bp direct repeats followed by an open reading frame encoding a 39K basic protein.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramhill D., Kornberg A. A model for initiation at origins of DNA replication. Cell. 1988 Sep 23;54(7):915–918. doi: 10.1016/0092-8674(88)90102-x. [DOI] [PubMed] [Google Scholar]
- Brunton J., Bennett P., Grinsted J. Molecular nature of a plasmid specifying beta-lactamase production in Haemophilus ducreyi. J Bacteriol. 1981 Dec;148(3):788–795. doi: 10.1128/jb.148.3.788-795.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton J., Meier M., Ehrman N., Maclean I., Slaney L., Albritton W. L. Molecular epidemiology of beta-lactamase-specifying plasmids of Haemophilus ducreyi. Antimicrob Agents Chemother. 1982 Jun;21(6):857–863. doi: 10.1128/aac.21.6.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton J., Meier M., Erhman N., Clare D., Almawy R. Origin of small beta-lactamase-specifying plasmids in Haemophilus species and Neisseria gonorrhoeae. J Bacteriol. 1986 Oct;168(1):374–379. doi: 10.1128/jb.168.1.374-379.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattoraj D. K., Snyder K. M., Abeles A. L. P1 plasmid replication: multiple functions of RepA protein at the origin. Proc Natl Acad Sci U S A. 1985 May;82(9):2588–2592. doi: 10.1073/pnas.82.9.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. T., Clowes R. C. Nucleotide sequence comparisons of plasmids pHD131, pJB1, pFA3, and pFA7 and beta-lactamase expression in Escherichia coli, Haemophilus influenzae, and Neisseria gonorrhoeae. J Bacteriol. 1987 Jul;169(7):3124–3130. doi: 10.1128/jb.169.7.3124-3130.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danner D. B., Pifer M. L. Plasmid cloning vectors resistant to ampicillin and tetracycline which can replicate in both E. coli and Haemophilus cells. Gene. 1982 Apr;18(1):101–105. doi: 10.1016/0378-1119(82)90062-2. [DOI] [PubMed] [Google Scholar]
- Dickgiesser N., Bennett P. M., Richmond M. H. Penicillinase-producing Neisseria gonorrhoeae: a molecular comparison of 5.3-kb and 7.4-kb beta-lactamase plasmids. J Bacteriol. 1982 Sep;151(3):1171–1175. doi: 10.1128/jb.151.3.1171-1175.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon J. A., Yeung K. H. Beta-lactamase plasmids and chromosomally mediated antibiotic resistance in pathogenic Neisseria species. Clin Microbiol Rev. 1989 Apr;2 (Suppl):S125–S133. doi: 10.1128/cmr.2.suppl.s125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon J. R., Pauzé M., Yeung K. H. Spread of penicillinase-producing and transfer plasmids from the gonococcus to Neisseria meningitidis. Lancet. 1983 Apr 9;1(8328):779–781. doi: 10.1016/s0140-6736(83)91846-9. [DOI] [PubMed] [Google Scholar]
- Gill R. E., Heffron F., Falkow S. Identification of the protein encoded by the transposable element Tn3 which is required for its transposition. Nature. 1979 Dec 20;282(5741):797–801. doi: 10.1038/282797a0. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Huang A., de Grandis S., Friesen J., Karmali M., Petric M., Congi R., Brunton J. L. Cloning and expression of the genes specifying Shiga-like toxin production in Escherichia coli H19. J Bacteriol. 1986 May;166(2):375–379. doi: 10.1128/jb.166.2.375-379.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley W., Bastia D. Replication initiator protein of plasmid R6K autoregulates its own synthesis at the transcriptional step. Proc Natl Acad Sci U S A. 1985 May;82(9):2574–2578. doi: 10.1073/pnas.82.9.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McNicol P. J., Albritton W. L., Ronald A. R. Characterization of ampicillin resistance plasmids of Haemophilus ducreyi and Neisseria gonorrhoeae with regard to location of origin of transfer and mobilization by a conjugative plasmid of Haemophilus ducreyi. J Bacteriol. 1983 Oct;156(1):437–440. doi: 10.1128/jb.156.1.437-440.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNicol P. J., Albritton W. L., Ronald A. R. Origin and direction of in vitro replication of Haemophilus ducreyi and Neisseria gonorrhoeae ampicillin resistance plasmids. J Bacteriol. 1984 Apr;158(1):393–395. doi: 10.1128/jb.158.1.393-395.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Prentki P., Krisch H. M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984 Sep;29(3):303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- Rubens C., Heffron F., Falkow S. Transposition of a plasmid deoxyribonucleic acid sequence that mediates ampicillin resistance: independence from host rec functions and orientation of insertion. J Bacteriol. 1976 Oct;128(1):425–434. doi: 10.1128/jb.128.1.425-434.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. Determinant of cistron specificity in bacterial ribosomes. Nature. 1975 Mar 6;254(5495):34–38. doi: 10.1038/254034a0. [DOI] [PubMed] [Google Scholar]
- Sox T. E., Mohammed W., Sparling P. F. Transformation-derived Neisseria gonorrhoeae plasmids with altered structure and function. J Bacteriol. 1979 May;138(2):510–518. doi: 10.1128/jb.138.2.510-518.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein D. C., Young F. E., Tenover F. C., Clark V. L. Characterization of a chimeric beta-lactamase plasmid of Neisseria gonorrhoeae which can function in Escherichia coli. Mol Gen Genet. 1983;189(1):77–84. doi: 10.1007/BF00326058. [DOI] [PubMed] [Google Scholar]
- Tomb J. F., Barcak G. J., Chandler M. S., Redfield R. J., Smith H. O. Transposon mutagenesis, characterization, and cloning of transformation genes of Haemophilus influenzae Rd. J Bacteriol. 1989 Jul;171(7):3796–3802. doi: 10.1128/jb.171.7.3796-3802.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Yeung K. H., Dillon J. A. Construction of miniplasmids from the 7.2-kb and 5.1-kb penicillinase-producing plasmids of Neisseria gonorrhoeae reveals two replication regions. Plasmid. 1988 Nov;20(3):232–240. doi: 10.1016/0147-619x(88)90029-7. [DOI] [PubMed] [Google Scholar]
- Yeung K. H., Dillon J. R., Pauzé M., Wallace E. A novel 4.9-kilobase plasmid associated with an outbreak of penicillinase-producing Neisseria gonorrhoeae. J Infect Dis. 1986 Jun;153(6):1162–1165. doi: 10.1093/infdis/153.6.1162. [DOI] [PubMed] [Google Scholar]