Abstract
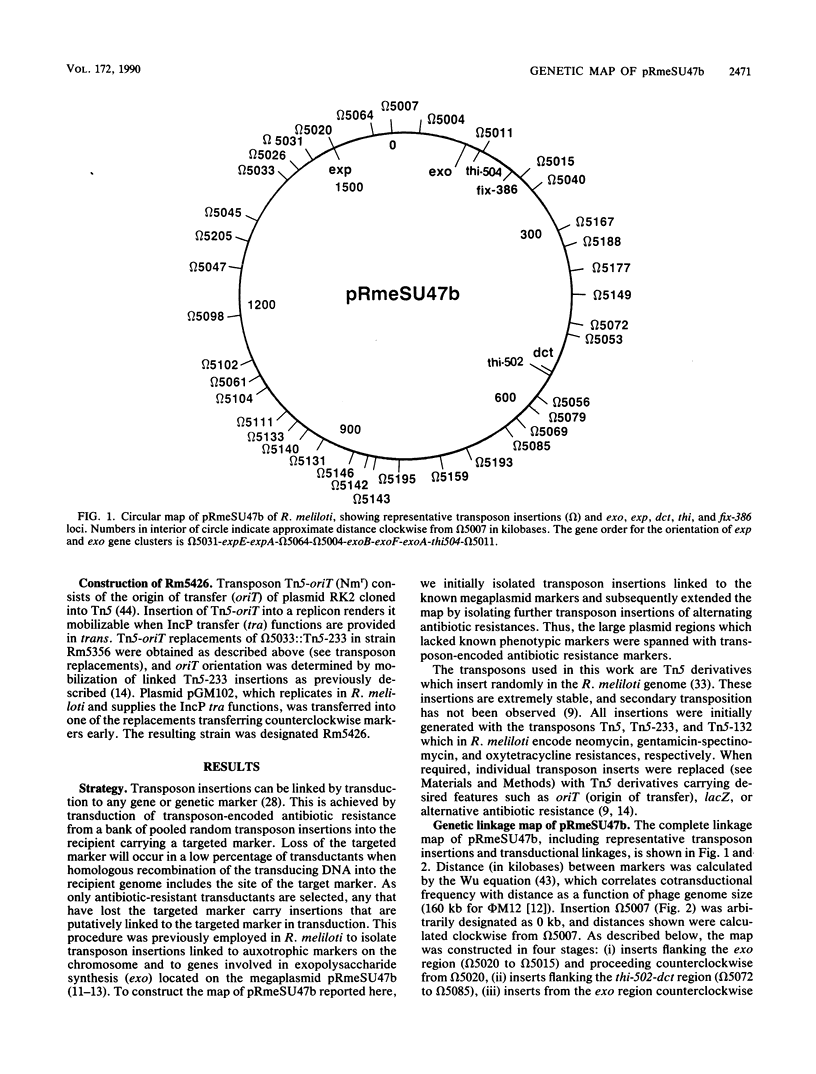
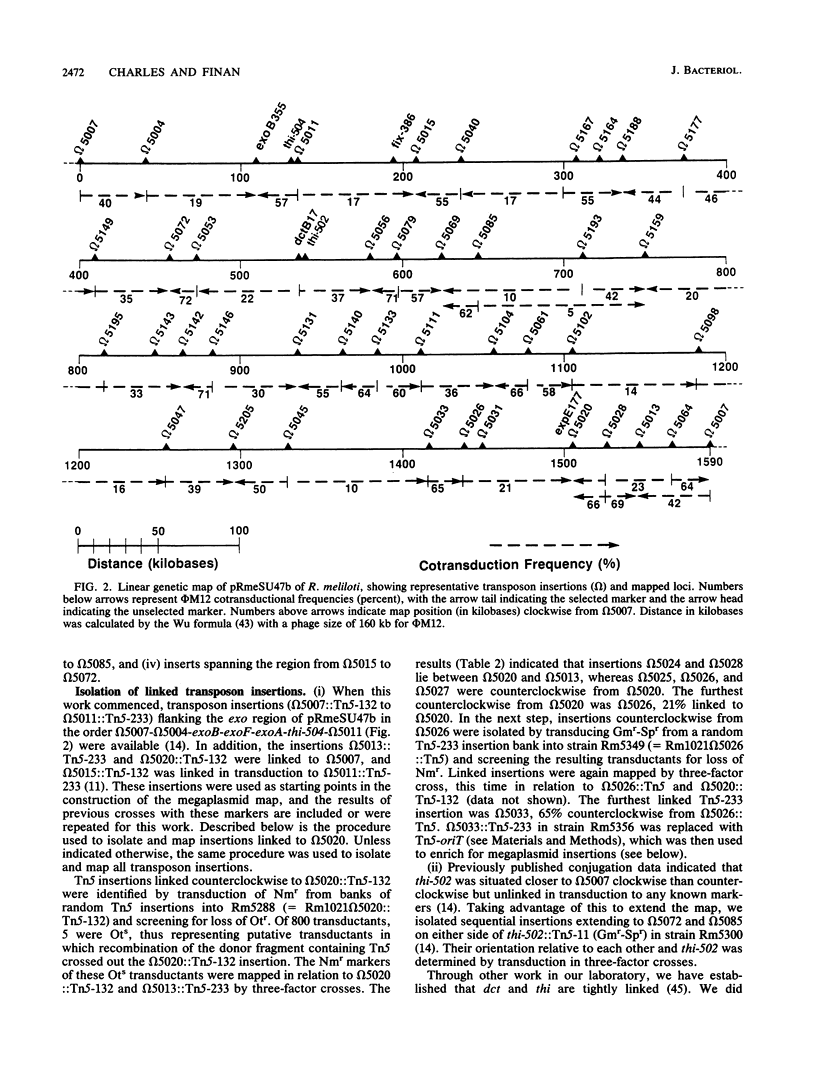
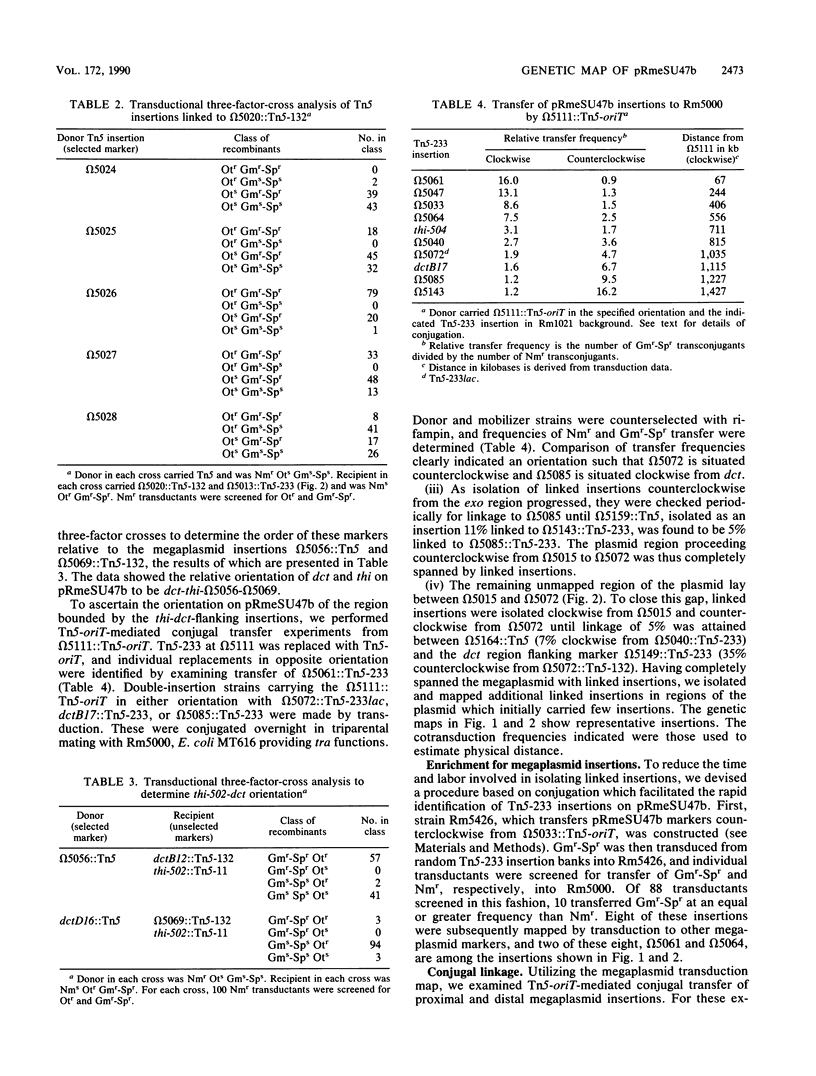
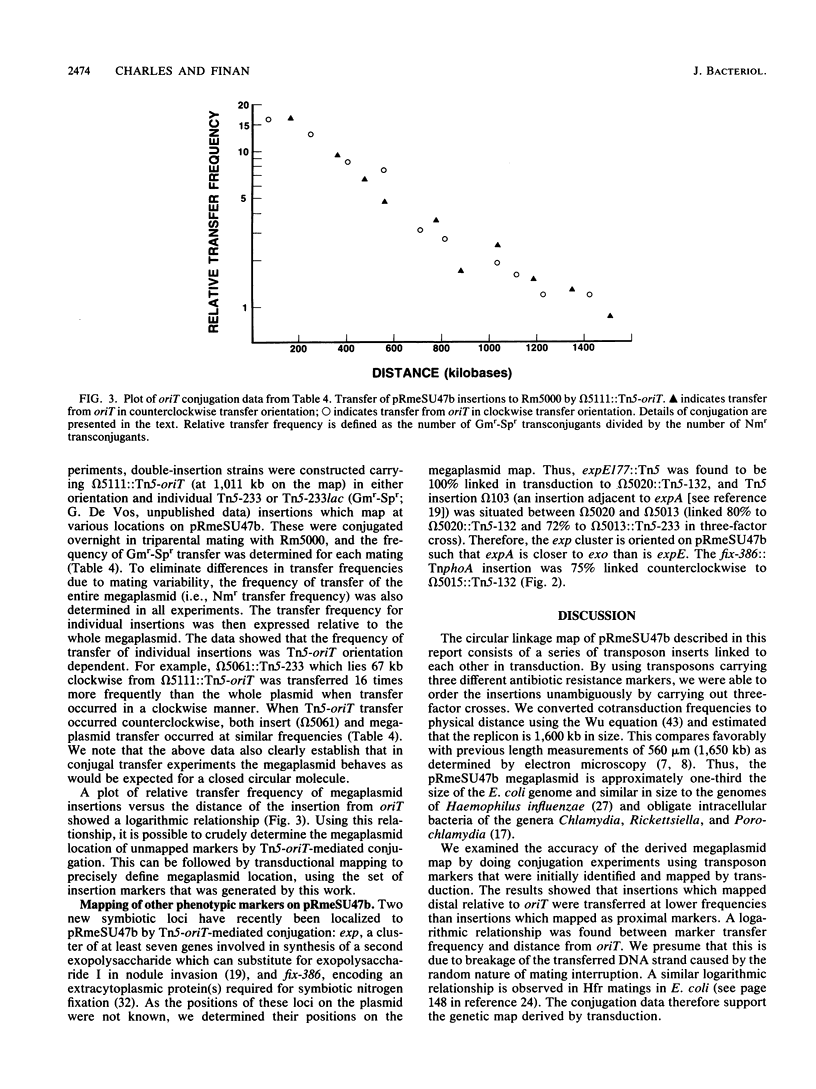
A circular linkage map of the Rhizobium meliloti megaplasmid pRmeSU47b was constructed. The map consists of transposon insertions carrying alternating antibiotic resistance markers linked by phi M12 transduction. Data from conjugation experiments utilizing donor strains carrying Tn5-oriT insertions in the megaplasmid supported the proposed genetic map. In addition, the positions of previously identified Fix, exopolysaccharide synthetic, thiamine synthetic, and C4-dicarboxylate transport loci on the megaplasmid map were determined. By converting cotransduction frequencies to physical distance, we calculated the replicon to be 1,600 kilobases in size, which compares favorably with previous physical estimates.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avery L., Kaiser D. In situ transposon replacement and isolation of a spontaneous tandem genetic duplication. Mol Gen Genet. 1983;191(1):99–109. doi: 10.1007/BF00330896. [DOI] [PubMed] [Google Scholar]
- Banfalvi Z., Kondorosi E., Kondorosi A. Rhizobium meliloti carries two megaplasmids. Plasmid. 1985 Mar;13(2):129–138. doi: 10.1016/0147-619x(85)90065-4. [DOI] [PubMed] [Google Scholar]
- Burkardt B., Burkardt H. J. Visualization and exact molecular weight determination of a Rhizobium meliloti megaplasmid. J Mol Biol. 1984 May 15;175(2):213–218. doi: 10.1016/0022-2836(84)90475-3. [DOI] [PubMed] [Google Scholar]
- Burkardt B., Schillik D., Pühler A. Physical characterization of Rhizobium meliloti megaplasmids. Plasmid. 1987 Jan;17(1):13–25. doi: 10.1016/0147-619x(87)90004-7. [DOI] [PubMed] [Google Scholar]
- De Vos G. F., Walker G. C., Signer E. R. Genetic manipulations in Rhizobium meliloti utilizing two new transposon Tn5 derivatives. Mol Gen Genet. 1986 Sep;204(3):485–491. doi: 10.1007/BF00331029. [DOI] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan T. M. Genetic and physical analyses of group E exo- mutants of Rhizobium meliloti. J Bacteriol. 1988 Jan;170(1):474–477. doi: 10.1128/jb.170.1.474-477.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan T. M., Hartweig E., LeMieux K., Bergman K., Walker G. C., Signer E. R. General transduction in Rhizobium meliloti. J Bacteriol. 1984 Jul;159(1):120–124. doi: 10.1128/jb.159.1.120-124.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan T. M., Hirsch A. M., Leigh J. A., Johansen E., Kuldau G. A., Deegan S., Walker G. C., Signer E. R. Symbiotic mutants of Rhizobium meliloti that uncouple plant from bacterial differentiation. Cell. 1985 Apr;40(4):869–877. doi: 10.1016/0092-8674(85)90346-0. [DOI] [PubMed] [Google Scholar]
- Finan T. M., Kunkel B., De Vos G. F., Signer E. R. Second symbiotic megaplasmid in Rhizobium meliloti carrying exopolysaccharide and thiamine synthesis genes. J Bacteriol. 1986 Jul;167(1):66–72. doi: 10.1128/jb.167.1.66-72.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan T. M., Oresnik I., Bottacin A. Mutants of Rhizobium meliloti defective in succinate metabolism. J Bacteriol. 1988 Aug;170(8):3396–3403. doi: 10.1128/jb.170.8.3396-3403.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouts K. E., Barbour S. D. Insertion of transposons through the major cotransduction gap of Escherichia coli K-12. J Bacteriol. 1982 Jan;149(1):106–113. doi: 10.1128/jb.149.1.106-113.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frutos R., Pages M., Bellis M., Roizes G., Bergoin M. Pulsed-field gel electrophoresis determination of the genome size of obligate intracellular bacteria belonging to the genera Chlamydia, Rickettsiella, and Porochlamydia. J Bacteriol. 1989 Aug;171(8):4511–4513. doi: 10.1128/jb.171.8.4511-4513.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantotti B. V., Beer S. V. Plasmid-borne determinants of pigmentation and thiamine prototrophy in Erwinia herbicola. J Bacteriol. 1982 Sep;151(3):1627–1629. doi: 10.1128/jb.151.3.1627-1629.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazebrook J., Walker G. C. A novel exopolysaccharide can function in place of the calcofluor-binding exopolysaccharide in nodulation of alfalfa by Rhizobium meliloti. Cell. 1989 Feb 24;56(4):661–672. doi: 10.1016/0092-8674(89)90588-6. [DOI] [PubMed] [Google Scholar]
- Hansen J. B., Olsen R. H. Isolation of large bacterial plasmids and characterization of the P2 incompatibility group plasmids pMG1 and pMG5. J Bacteriol. 1978 Jul;135(1):227–238. doi: 10.1128/jb.135.1.227-238.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogrefe C., Friedrich B. Isolation and characterization of megaplasmid DNA from lithoautotrophic bacteria. Plasmid. 1984 Nov;12(3):161–169. doi: 10.1016/0147-619x(84)90040-4. [DOI] [PubMed] [Google Scholar]
- Jacoby G. A., Sutton L., Knobel L., Mammen P. Properties of IncP-2 plasmids of Pseudomonas spp. Antimicrob Agents Chemother. 1983 Aug;24(2):168–175. doi: 10.1128/aac.24.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauc L., Mitchell M., Goodgal S. H. Size and physical map of the chromosome of Haemophilus influenzae. J Bacteriol. 1989 May;171(5):2474–2479. doi: 10.1128/jb.171.5.2474-2479.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N., Roth J., Botstein D. Genetic engineering in vivo using translocatable drug-resistance elements. New methods in bacterial genetics. J Mol Biol. 1977 Oct 15;116(1):125–159. doi: 10.1016/0022-2836(77)90123-1. [DOI] [PubMed] [Google Scholar]
- Klintworth R., Husemann M., Salnikow J., Bowien B. Chromosomal and plasmid locations for phosphoribulokinase genes in Alcaligenes eutrophus. J Bacteriol. 1985 Nov;164(2):954–956. doi: 10.1128/jb.164.2.954-956.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh J. A., Signer E. R., Walker G. C. Exopolysaccharide-deficient mutants of Rhizobium meliloti that form ineffective nodules. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6231–6235. doi: 10.1073/pnas.82.18.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long S., McCune S., Walker G. C. Symbiotic loci of Rhizobium meliloti identified by random TnphoA mutagenesis. J Bacteriol. 1988 Sep;170(9):4257–4265. doi: 10.1128/jb.170.9.4257-4265.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade H. M., Long S. R., Ruvkun G. B., Brown S. E., Ausubel F. M. Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon Tn5 mutagenesis. J Bacteriol. 1982 Jan;149(1):114–122. doi: 10.1128/jb.149.1.114-122.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade H. M., Signer E. R. Genetic mapping of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1977 May;74(5):2076–2078. doi: 10.1073/pnas.74.5.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy P. J., Heycke N., Banfalvi Z., Tate M. E., de Bruijn F., Kondorosi A., Tempé J., Schell J. Genes for the catabolism and synthesis of an opine-like compound in Rhizobium meliloti are closely linked and on the Sym plasmid. Proc Natl Acad Sci U S A. 1987 Jan;84(2):493–497. doi: 10.1073/pnas.84.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince A. S., Barlam T. Isolation of a DNA fragment containing replication functions from IncP2 megaplasmid pMG2. J Bacteriol. 1985 Feb;161(2):792–794. doi: 10.1128/jb.161.2.792-794.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg C., Casse-Delbart F., Dusha I., David M., Boucher C. Megaplasmids in the plant-associated bacteria Rhizobium meliloti and Pseudomonas solanacearum. J Bacteriol. 1982 Apr;150(1):402–406. doi: 10.1128/jb.150.1.402-406.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römermann D., Friedrich B. Denitrification by Alcaligenes eutrophus is plasmid dependent. J Bacteriol. 1985 May;162(2):852–854. doi: 10.1128/jb.162.2.852-854.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M., Baker T. A., Schnitzler G., Deischel S. M., Goel M., Dove W., Jaacks K. J., Grossman A. D., Erickson J. W., Gross C. A. A collection of strains containing genetically linked alternating antibiotic resistance elements for genetic mapping of Escherichia coli. Microbiol Rev. 1989 Mar;53(1):1–24. doi: 10.1128/mr.53.1.1-24.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger L., Ziegler S. F., Huffman G. A., Knauf V. C., Peet R., Moore L. W., Gordon M. P., Nester E. W. New class of limited-host-range Agrobacterium mega-tumor-inducing plasmids lacking homology to the transferred DNA of a wide-host-range, tumor-inducing plasmid. J Bacteriol. 1985 Nov;164(2):723–730. doi: 10.1128/jb.164.2.723-730.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson R. J., Chan Y. K., Wheatcroft R., Yang A. F., Han S. H. Rhizobium meliloti genes required for C4-dicarboxylate transport and symbiotic nitrogen fixation are located on a megaplasmid. J Bacteriol. 1988 Feb;170(2):927–934. doi: 10.1128/jb.170.2.927-934.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T. T. A model for three-point analysis of random general transduction. Genetics. 1966 Aug;54(2):405–410. doi: 10.1093/genetics/54.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakobson E. A., Guiney D. G., Jr Conjugal transfer of bacterial chromosomes mediated by the RK2 plasmid transfer origin cloned into transposon Tn5. J Bacteriol. 1984 Oct;160(1):451–453. doi: 10.1128/jb.160.1.451-453.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarosh O. K., Charles T. C., Finan T. M. Analysis of C4-dicarboxylate transport genes in Rhizobium meliloti. Mol Microbiol. 1989 Jun;3(6):813–823. doi: 10.1111/j.1365-2958.1989.tb00230.x. [DOI] [PubMed] [Google Scholar]


