Abstract
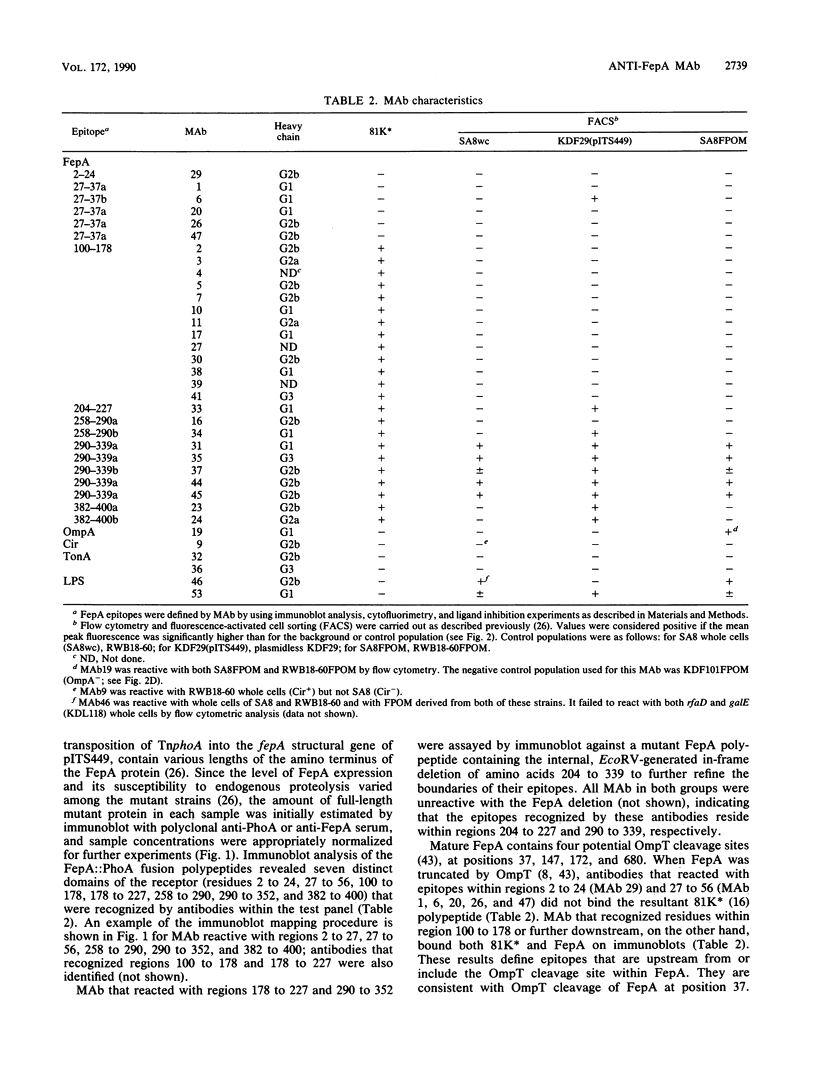
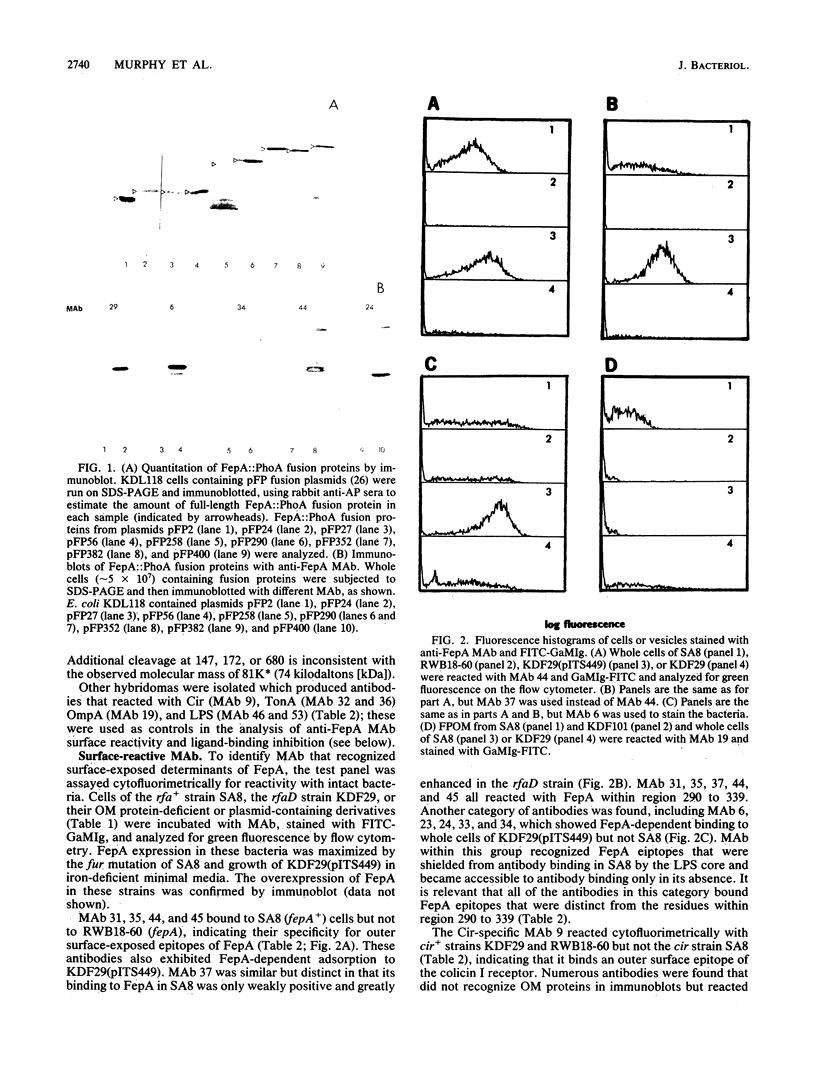
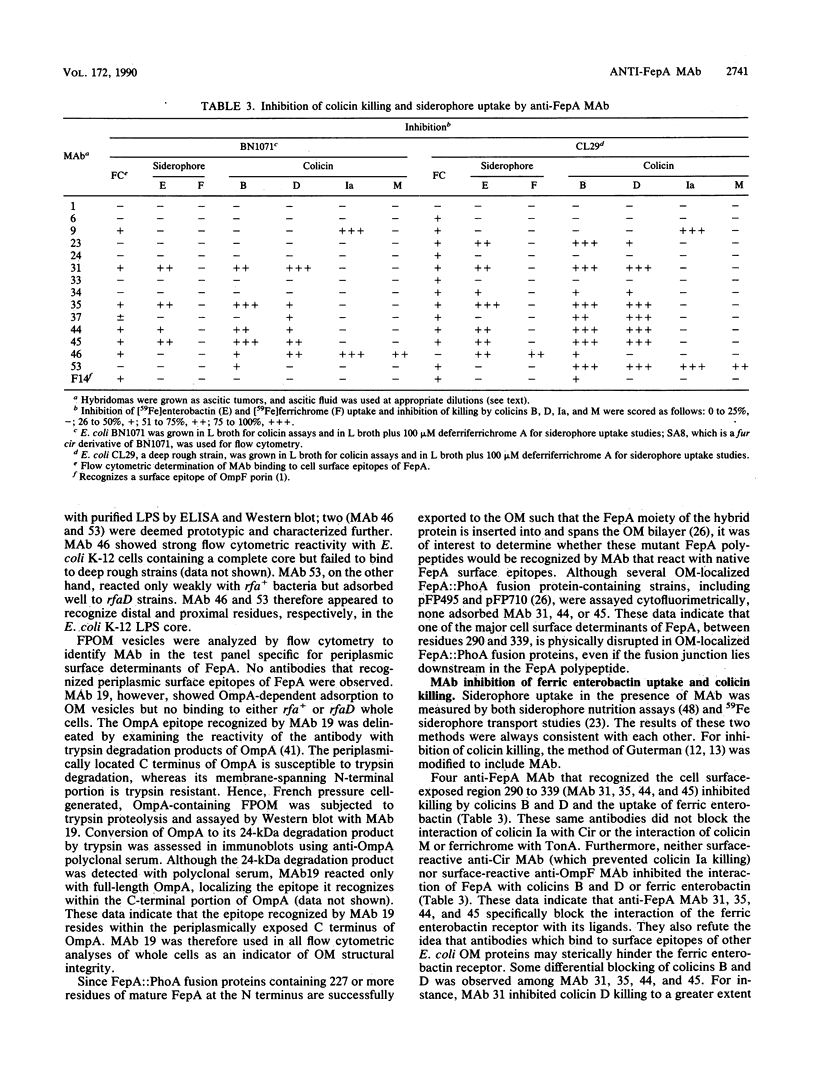
Monoclonal antibodies (MAb) were raised to the Escherichia coli K-12 ferric enterobactin receptor, FepA, and used to identify regions of the polypeptide that are involved in interaction with its ligands ferric enterobactin and colicins B and D. A total of 11 distinct FepA epitopes were identified. The locations of these epitopes within the primary sequence of FepA were mapped by screening MAb against a library of FepA::PhoA fusion proteins, a FepA deletion mutant, and proteolytically modified FepA. These experiments localized the 11 epitopes to seven different regions within the FepA polypeptide, including residues 2 to 24, 27 to 37, 100 to 178, 204 to 227, 258 to 290, 290 to 339, and 382 to 400 of the mature protein. Cell surface-exposed epitopes of FepA were identified and discriminated by cytofluorimetry and by the ability of MAb that recognize them to block the interaction of FepA with its ligands. Seven surface epitopes were defined, including one each in regions 27 to 37, 204 to 227, and 258 to 290 and two each in regions 290 to 339 and 382 to 400. One of these, within region 290 to 339, was recognized by MAb in bacteria containing intact (rfa+) lipopolysaccharide (LPS); all other surface epitopes were susceptible to MAb binding only in a strain containing a truncated (rfaD) LPS core, suggesting that they are physically shielded by E. coli K-12 LPS core sugars. Antibody binding to FepA surface epitopes within region 290 to 339 or 382 to 400 inhibited killing by colicin B or D and the uptake of ferric enterobactin. In addition to the FepA-specific MAb, antibodies that recognized other outer membrane components, including Cir, OmpA, TonA, and LPS, were identified. Immunochemical and biochemical characterization of the surface structures of FepA and analysis of its hydrophobicity and amphilicity were used to generate a model of the ferric enterobactin receptor's transmembrane strands, surface peptides, and ligand-binding domains.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bentley A. T., Klebba P. E. Effect of lipopolysaccharide structure on reactivity of antiporin monoclonal antibodies with the bacterial cell surface. J Bacteriol. 1988 Mar;170(3):1063–1068. doi: 10.1128/jb.170.3.1063-1068.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Charbit A., Clement J. M., Hofnung M. Further sequence analysis of the phage lambda receptor site. Possible implications for the organization of the lamB protein in Escherichia coli K12. J Mol Biol. 1984 May 25;175(3):395–401. doi: 10.1016/0022-2836(84)90355-3. [DOI] [PubMed] [Google Scholar]
- Coleman W. G., Jr, Leive L. Two mutations which affect the barrier function of the Escherichia coli K-12 outer membrane. J Bacteriol. 1979 Sep;139(3):899–910. doi: 10.1128/jb.139.3.899-910.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csonka L. N., Clark A. J. Construction of an Hfr strain useful for transferring recA mutations between Escherichia coli strains. J Bacteriol. 1980 Jul;143(1):529–530. doi: 10.1128/jb.143.1.529-530.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg D. Three-dimensional structure of membrane and surface proteins. Annu Rev Biochem. 1984;53:595–623. doi: 10.1146/annurev.bi.53.070184.003115. [DOI] [PubMed] [Google Scholar]
- Eisenberg D., Weiss R. M., Terwilliger T. C. The hydrophobic moment detects periodicity in protein hydrophobicity. Proc Natl Acad Sci U S A. 1984 Jan;81(1):140–144. doi: 10.1073/pnas.81.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiss E. H., Stanley-Samuelson P., Neilands J. B. Properties and proteolysis of ferric enterobactin outer membrane receptor in Escherichia coli K12. Biochemistry. 1982 Aug 31;21(18):4517–4522. doi: 10.1021/bi00261a050. [DOI] [PubMed] [Google Scholar]
- Gabay J., Benson S., Schwartz M. Genetic mapping of antigenic determinants on a membrane protein. J Biol Chem. 1983 Feb 25;258(4):2410–2414. [PubMed] [Google Scholar]
- Garavito R. M., Jenkins J., Jansonius J. N., Karlsson R., Rosenbusch J. P. X-ray diffraction analysis of matrix porin, an integral membrane protein from Escherichia coli outer membranes. J Mol Biol. 1983 Feb 25;164(2):313–327. doi: 10.1016/0022-2836(83)90079-7. [DOI] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Guterman S. K., Dann L. Excretion of enterochelin by exbA and exbB mutants of Escherichia coli. J Bacteriol. 1973 Jun;114(3):1225–1230. doi: 10.1128/jb.114.3.1225-1230.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guterman S. K., Dann L. Excretion of enterochelin by exbA and exbB mutants of Escherichia coli. J Bacteriol. 1973 Jun;114(3):1225–1230. doi: 10.1128/jb.114.3.1225-1230.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guterman S. K. Inhibition of colicin B by enterochelin. Biochem Biophys Res Commun. 1971 Sep;44(5):1149–1155. doi: 10.1016/s0006-291x(71)80206-1. [DOI] [PubMed] [Google Scholar]
- Heller K. B. Apparent molecular weights of a heat-modifiable protein from the outer membrane of Escherichia coli in gels with different acrylamide concentrations. J Bacteriol. 1978 Jun;134(3):1181–1183. doi: 10.1128/jb.134.3.1181-1183.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollifield W. C., Jr, Fiss E. H., Neilands J. B. Modification of a ferric enterobactin receptor protein from the outer membrane of Escherichia coli. Biochem Biophys Res Commun. 1978 Jul 28;83(2):739–746. doi: 10.1016/0006-291x(78)91051-3. [DOI] [PubMed] [Google Scholar]
- Jap B. K. High-resolution electron diffraction of reconstituted PhoE porin. J Mol Biol. 1988 Jan 5;199(1):229–231. doi: 10.1016/0022-2836(88)90393-2. [DOI] [PubMed] [Google Scholar]
- Klebba P. E., McIntosh M. A., Neilands J. B. Kinetics of biosynthesis of iron-regulated membrane proteins in Escherichia coli. J Bacteriol. 1982 Mar;149(3):880–888. doi: 10.1128/jb.149.3.880-888.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lundrigan M. D., Kadner R. J. Nucleotide sequence of the gene for the ferrienterochelin receptor FepA in Escherichia coli. Homology among outer membrane receptors that interact with TonB. J Biol Chem. 1986 Aug 15;261(23):10797–10801. [PubMed] [Google Scholar]
- Manning P. A., Puspurs A., Reeves P. Outer membrane of Escherichia coli K-12: isolation of mutants with altered protein 3A by using host range mutants of bacteriophage K3. J Bacteriol. 1976 Sep;127(3):1080–1084. doi: 10.1128/jb.127.3.1080-1084.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh M. A., Chenault S. S., Earhart C. F. Genetic and physiological studies on the relationship between colicin B resistance and ferrienterochelin uptake in Escherichia coli K-12. J Bacteriol. 1979 Jan;137(1):653–657. doi: 10.1128/jb.137.1.653-657.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima S. Effect of sodium dodecylsulfate and heating on protein conformation in outer and cytoplasmic membranes from Escherichia coli. Biochem Biophys Res Commun. 1974 Dec 23;61(4):1221–1226. doi: 10.1016/s0006-291x(74)80414-6. [DOI] [PubMed] [Google Scholar]
- Murphy C. K., Klebba P. E. Export of FepA::PhoA fusion proteins to the outer membrane of Escherichia coli K-12. J Bacteriol. 1989 Nov;171(11):5894–5900. doi: 10.1128/jb.171.11.5894-5900.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabedryk E., Garavito R. M., Breton J. The orientation of beta-sheets in porin. A polarized Fourier transform infrared spectroscopic investigation. Biophys J. 1988 May;53(5):671–676. doi: 10.1016/S0006-3495(88)83148-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Mizushima S. Effects of heating in dodecyl sulfate solution on the conformation and electrophoretic mobility of isolated major outer membrane proteins from Escherichia coli K-12. J Biochem. 1976 Dec;80(6):1411–1422. doi: 10.1093/oxfordjournals.jbchem.a131414. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olschläger T., Schramm E., Braun V. Cloning and expression of the activity and immunity genes of colicins B and M on ColBM plasmids. Mol Gen Genet. 1984;196(3):482–487. doi: 10.1007/BF00436196. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P., Reeves P. Characterization of group B colicin-resistant mutants of Escherichia coli K-12: colicin resistance and the role of enterochelin. J Bacteriol. 1976 Jul;127(1):218–228. doi: 10.1128/jb.127.1.218-228.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley A. P., Reeves P. Comparison of colicins B-K260 and D-CA23: purification and characterization of the colicins and examination of colicin immunity in the producing strains. Antimicrob Agents Chemother. 1977 Feb;11(2):345–358. doi: 10.1128/aac.11.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. C., DeAntonio L., Eisenberg D. Hydrophobic organization of membrane proteins. Science. 1989 Aug 4;245(4917):510–513. doi: 10.1126/science.2667138. [DOI] [PubMed] [Google Scholar]
- Reithmeier R. A., Bragg P. D. Molecular characterization of a heat-modifiable protein from the outer membrane of Escherichia coli. Arch Biochem Biophys. 1977 Jan 30;178(2):527–534. doi: 10.1016/0003-9861(77)90223-5. [DOI] [PubMed] [Google Scholar]
- Rosenbusch J. P. Characterization of the major envelope protein from Escherichia coli. Regular arrangement on the peptidoglycan and unusual dodecyl sulfate binding. J Biol Chem. 1974 Dec 25;249(24):8019–8029. [PubMed] [Google Scholar]
- Schenkman S., Couture E., Schwartz M. Monoclonal antibodies reveal lamB antigenic determinants on both faces of the Escherichia coli outer membrane. J Bacteriol. 1983 Sep;155(3):1382–1392. doi: 10.1128/jb.155.3.1382-1392.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Outer membrane proteins of Escherichia coli. I. Effect of preparative conditions on the migration of protein in polyacrylamide gels. Arch Biochem Biophys. 1973 Aug;157(2):541–552. doi: 10.1016/0003-9861(73)90673-5. [DOI] [PubMed] [Google Scholar]
- Schweizer M., Hindennach I., Garten W., Henning U. Major proteins of the Escherichia coli outer cell envelope membrane. Interaction of protein II with lipopolysaccharide. Eur J Biochem. 1978 Jan 2;82(1):211–217. doi: 10.1111/j.1432-1033.1978.tb12013.x. [DOI] [PubMed] [Google Scholar]
- Shinnick T. M., Sutcliffe J. G., Green N., Lerner R. A. Synthetic peptide immunogens as vaccines. Annu Rev Microbiol. 1983;37:425–446. doi: 10.1146/annurev.mi.37.100183.002233. [DOI] [PubMed] [Google Scholar]
- Sugimura K., Higashi N. A novel outer-membrane-associated protease in Escherichia coli. J Bacteriol. 1988 Aug;170(8):3650–3654. doi: 10.1128/jb.170.8.3650-3654.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmis K. Purification and characterization of colicin D. J Bacteriol. 1972 Jan;109(1):12–20. doi: 10.1128/jb.109.1.12-20.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommassen J., Lugtenberg B. Amino terminus of outer membrane PhoE protein: localization by use of a bla-phoE hybrid gene. J Bacteriol. 1984 Jan;157(1):327–329. doi: 10.1128/jb.157.1.327-329.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel H., Jähnig F. Models for the structure of outer-membrane proteins of Escherichia coli derived from raman spectroscopy and prediction methods. J Mol Biol. 1986 Jul 20;190(2):191–199. doi: 10.1016/0022-2836(86)90292-5. [DOI] [PubMed] [Google Scholar]
- Wang C. C., Newton A. An additional step in the transport of iron defined by the tonB locus of Escherichia coli. J Biol Chem. 1971 Apr 10;246(7):2147–2151. [PubMed] [Google Scholar]
- Wayne R., Frick K., Neilands J. B. Siderophore protection against colicins M, B, V, and Ia in Escherichia coli. J Bacteriol. 1976 Apr;126(1):7–12. doi: 10.1128/jb.126.1.7-12.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmot C. M., Thornton J. M. Analysis and prediction of the different types of beta-turn in proteins. J Mol Biol. 1988 Sep 5;203(1):221–232. doi: 10.1016/0022-2836(88)90103-9. [DOI] [PubMed] [Google Scholar]