Abstract
The nucleotide sequence of the Escherichia coli K-12 recG gene was determined. recG was identified as an open reading frame located between the spoT operon and the convergent gltS gene. It encodes a polypeptide of 693 amino acids which was identified as a 76-kDa protein by sodium dodecyl sulfate-polyacrylamide gel electrophoresis after it was labeled with [35S]methionine in maxicells. The sequence determined revealed no obvious promoter. Synthesis of RecG by plasmids carrying the intact gene varied with the orientation of the insert relative to the vector promoter and with the extent of upstream spoT operon sequence included in the construction. It is concluded that recG is the fourth and last gene in the spoT operon, although a possible promoter for independent transcription of spoU and recG was identified near the end of the spoT gene. The primary sequence of RecG revealed that it is related to proteins that act as helicases and has a well-conserved motif identified with ATP binding.
Full text
PDF

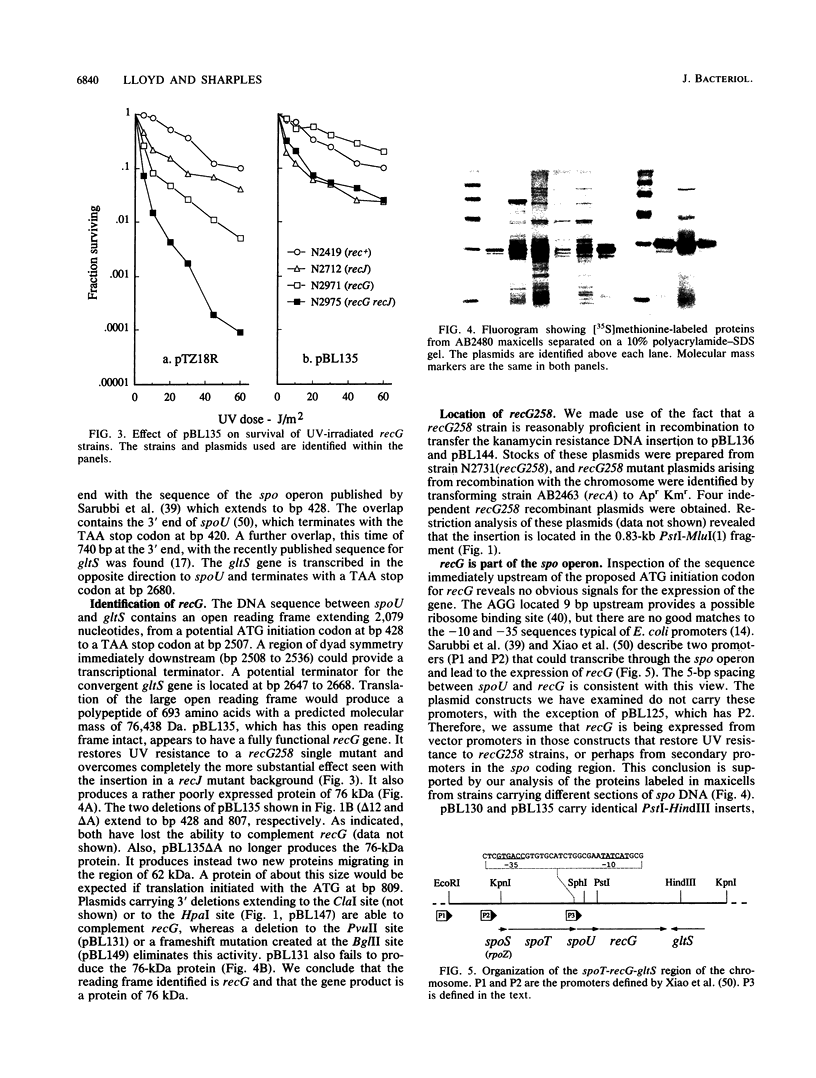



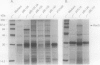
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armengod M. E., García-Sogo M., Lambíes E. Transcriptional organization of the dnaN and recF genes of Escherichia coli K-12. J Biol Chem. 1988 Aug 25;263(24):12109–12114. [PubMed] [Google Scholar]
- Armengod M. E., Lambíes E. Overlapping arrangement of the recF and dnaN operons of Escherichia coli; positive and negative control sequences. Gene. 1986;43(3):183–196. doi: 10.1016/0378-1119(86)90206-4. [DOI] [PubMed] [Google Scholar]
- Arthur H. M., Bramhill D., Eastlake P. B., Emmerson P. T. Cloning of the uvrD gene of E. coli and identification of the product. Gene. 1982 Oct;19(3):285–295. doi: 10.1016/0378-1119(82)90018-x. [DOI] [PubMed] [Google Scholar]
- Benson F., Collier S., Lloyd R. G. Evidence of abortive recombination in ruv mutants of Escherichia coli K12. Mol Gen Genet. 1991 Feb;225(2):266–272. doi: 10.1007/BF00269858. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Bradley M. K., Smith T. F., Lathrop R. H., Livingston D. M., Webster T. A. Consensus topography in the ATP binding site of the simian virus 40 and polyomavirus large tumor antigens. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4026–4030. doi: 10.1073/pnas.84.12.4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly B., Parsons C. A., Benson F. E., Dunderdale H. J., Sharples G. J., Lloyd R. G., West S. C. Resolution of Holliday junctions in vitro requires the Escherichia coli ruvC gene product. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6063–6067. doi: 10.1073/pnas.88.14.6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford M. J., Anton I. A., Lane D. P. Nuclear protein with sequence homology to translation initiation factor eIF-4A. Nature. 1988 Apr 21;332(6166):736–738. doi: 10.1038/332736a0. [DOI] [PubMed] [Google Scholar]
- Gentry D. R., Burgess R. R. rpoZ, encoding the omega subunit of Escherichia coli RNA polymerase, is in the same operon as spoT. J Bacteriol. 1989 Mar;171(3):1271–1277. doi: 10.1128/jb.171.3.1271-1277.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A. E., Koonin E. V., Donchenko A. P., Blinov V. M. A conserved NTP-motif in putative helicases. Nature. 1988 May 5;333(6168):22–22. doi: 10.1038/333022a0. [DOI] [PubMed] [Google Scholar]
- Gorbalenya A. E., Koonin E. V., Donchenko A. P., Blinov V. M. Two related superfamilies of putative helicases involved in replication, recombination, repair and expression of DNA and RNA genomes. Nucleic Acids Res. 1989 Jun 26;17(12):4713–4730. doi: 10.1093/nar/17.12.4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley C. B., Reynolds R. P. Analysis of E. coli promoter sequences. Nucleic Acids Res. 1987 Mar 11;15(5):2343–2361. doi: 10.1093/nar/15.5.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgman T. C. A new superfamily of replicative proteins. Nature. 1988 May 5;333(6168):22–23. doi: 10.1038/333022b0. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P., Theriot L. Three loci in Escherichia coli K-12 that control the excision of pyrimidine dimers and certain other mutagen products from DNA. Genetics. 1966 Jun;53(6):1119–1136. doi: 10.1093/genetics/53.6.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalman M., Gentry D. R., Cashel M. Characterization of the Escherichia coli K12 gltS glutamate permease gene. Mol Gen Genet. 1991 Mar;225(3):379–386. doi: 10.1007/BF00261677. [DOI] [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- Lee E. H., Masai H., Allen G. C., Jr, Kornberg A. The priA gene encoding the primosomal replicative n' protein of Escherichia coli. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4620–4624. doi: 10.1073/pnas.87.12.4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Lloyd R. G., Barbour S. D. The genetic location of the sbcA gene of Escherichia coli. Mol Gen Genet. 1974;134(2):157–171. doi: 10.1007/BF00268417. [DOI] [PubMed] [Google Scholar]
- Lloyd R. G., Buckman C. Genetic analysis of the recG locus of Escherichia coli K-12 and of its role in recombination and DNA repair. J Bacteriol. 1991 Feb;173(3):1004–1011. doi: 10.1128/jb.173.3.1004-1011.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd R. G. Conjugational recombination in resolvase-deficient ruvC mutants of Escherichia coli K-12 depends on recG. J Bacteriol. 1991 Sep;173(17):5414–5418. doi: 10.1128/jb.173.17.5414-5418.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd R. G., Low B., Godson G. N., Birge E. A. Isolation and characterization of an Escherichia coli K-12 mutant with a temperature-sensitive recA- phenotype. J Bacteriol. 1974 Oct;120(1):407–415. doi: 10.1128/jb.120.1.407-415.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett S. T., Kolodner R. D. Nucleotide sequence of the Escherichia coli recJ chromosomal region and construction of recJ-overexpression plasmids. J Bacteriol. 1991 Jan;173(1):353–364. doi: 10.1128/jb.173.1.353-364.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maples V. F., Kushner S. R. DNA repair in Escherichia coli: identification of the uvrD gene product. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5616–5620. doi: 10.1073/pnas.79.18.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson S. W., Kaiser-Rogers K. A. DNA helicases. Annu Rev Biochem. 1990;59:289–329. doi: 10.1146/annurev.bi.59.070190.001445. [DOI] [PubMed] [Google Scholar]
- Morrison P. T., Lovett S. T., Gilson L. E., Kolodner R. Molecular analysis of the Escherichia coli recO gene. J Bacteriol. 1989 Jul;171(7):3641–3649. doi: 10.1128/jb.171.7.3641-3649.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muskavitch K. M., Linn S. A unified mechanism for the nuclease and unwinding activities of the recBC enzyme of Escherichia coli. J Biol Chem. 1982 Mar 10;257(5):2641–2648. [PubMed] [Google Scholar]
- Nielsen P. J., McMaster G. K., Trachsel H. Cloning of eukaryotic protein synthesis initiation factor genes: isolation and characterization of cDNA clones encoding factor eIF-4A. Nucleic Acids Res. 1985 Oct 11;13(19):6867–6880. doi: 10.1093/nar/13.19.6867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse P., DiGate R. J., Zavitz K. H., Marians K. J. Molecular cloning and DNA sequence analysis of Escherichia coli priA, the gene encoding the primosomal protein replication factor Y. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4615–4619. doi: 10.1073/pnas.87.12.4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeda K., Horiuchi T., Sekiguchi M. Molecular cloning of the uvrD gene of Escherichia coli that controls ultraviolet sensitivity and spontaneous mutation frequency. Mol Gen Genet. 1981;184(2):191–199. doi: 10.1007/BF00272904. [DOI] [PubMed] [Google Scholar]
- Radding C. M. Helical interactions in homologous pairing and strand exchange driven by RecA protein. J Biol Chem. 1991 Mar 25;266(9):5355–5358. [PubMed] [Google Scholar]
- Rosamond J., Telander K. M., Linn S. Modulation of the action of the recBC enzyme of Escherichia coli K-12 by Ca2+. J Biol Chem. 1979 Sep 10;254(17):8646–8652. [PubMed] [Google Scholar]
- Sancar A., Hack A. M., Rupp W. D. Simple method for identification of plasmid-coded proteins. J Bacteriol. 1979 Jan;137(1):692–693. doi: 10.1128/jb.137.1.692-693.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarubbi E., Rudd K. E., Xiao H., Ikehara K., Kalman M., Cashel M. Characterization of the spoT gene of Escherichia coli. J Biol Chem. 1989 Sep 5;264(25):15074–15082. [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. R. Homologous recombination in procaryotes. Microbiol Rev. 1988 Mar;52(1):1–28. doi: 10.1128/mr.52.1.1-28.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl F. W., Thomason L. C., Siddiqi I., Stahl M. M. Further tests of a recombination model in which chi removes the RecD subunit from the RecBCD enzyme of Escherichia coli. Genetics. 1990 Nov;126(3):519–533. doi: 10.1093/genetics/126.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm P. K., Hoekstra W. P., de Haan P. G., Verhoef C. Genetic recombination in Escherichia coli. IV. Isolation and characterization of recombination-deficiency mutants of Escherichia coli K12. Mutat Res. 1971 Sep;13(1):9–17. doi: 10.1016/0027-5107(71)90121-7. [DOI] [PubMed] [Google Scholar]
- Taylor A., Smith G. R. Unwinding and rewinding of DNA by the RecBC enzyme. Cell. 1980 Nov;22(2 Pt 2):447–457. doi: 10.1016/0092-8674(80)90355-4. [DOI] [PubMed] [Google Scholar]
- Umezu K., Nakayama K., Nakayama H. Escherichia coli RecQ protein is a DNA helicase. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5363–5367. doi: 10.1073/pnas.87.14.5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. E., Saraste M., Runswick M. J., Gay N. J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1(8):945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West S. C., Emmerson P. T. Induction of protein synthesis in Escherichia coli following UV- or gamma-irradiation, mitomycin C treatment or tif Expression. Mol Gen Genet. 1977 Feb 28;151(1):57–67. doi: 10.1007/BF00446913. [DOI] [PubMed] [Google Scholar]
- Xiao H., Kalman M., Ikehara K., Zemel S., Glaser G., Cashel M. Residual guanosine 3',5'-bispyrophosphate synthetic activity of relA null mutants can be eliminated by spoT null mutations. J Biol Chem. 1991 Mar 25;266(9):5980–5990. [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]