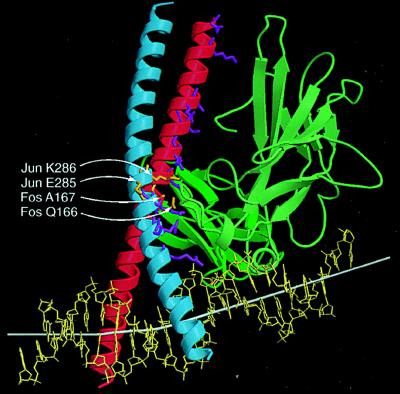
Figure 4.
Comparison of residues required for oriented heterodimer binding and cooperative DNA bending with contact residues in the x-ray crystal structure. The smoothed Cα trace of NFAT1 (green) and Fos–Jun (red-blue) are shown using ribbons to indicate α helices and arrows to indicate β sheets based on the x-ray crystal structure (24). The side chains of residues in Fos and Jun that are in close contact with NFAT1 in the x-ray crystal structure (24) are shown in stick representation. The subset of these residues that determined the orientation of heterodimer binding and cooperative DNA bending is shown in orange and indicated by white arrows. Other contact residues are shown in purple. The helix axis is shown by a white bar that is extended on both ends based on the three base pairs at the ends. The DNA helix axis was calculated using curves and the image was produced using molscript and raster3d.