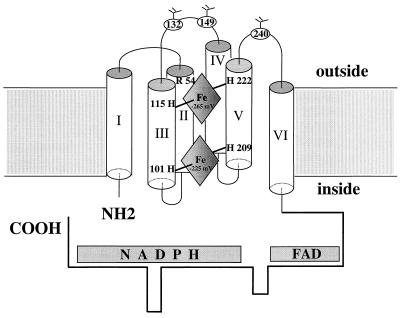
Figure 4.
Model for the location of the two nonidentical hemes in gp91phox. The six transmembranous helices and both NH2- and COOH-terminal tails are portrayed as indicated. The heme with Em of −265 mV is toward the outer face of the membrane and is coordinated possibly by His 115 and 222. The heme with Em of −225 mV is close to the inner face of the membrane and is coordinated by His 101 and 209. Arg 54 in helix II, which may react with a nearby propionate side chain of the heme (Em = −265 mV), also is shown. The three Asp residues at positions 132, 149, and 240 are indicated as glycosylation sites. Note that the N-terminal Methionine was counted as amino acid #1 in this model, thus explaining the difference between our numbering and that of Wallach et al. (38).