Abstract
We sought to determine whether mice deficient in the proinflammatory caspase-1, which cleaves precursors of IL-1β and IL-18, were protected against ischemic acute renal failure (ARF). Caspase-1–/– mice developed less ischemic ARF as judged by renal function and renal histology. These animals had significantly reduced blood urea nitrogen and serum creatinine levels and a lower morphological tubular necrosis score than did wild-type mice with ischemic ARF. Since caspase-1 activates IL-18, lack of mature IL-18 might protect these caspase-1–/– mice from ARF. In wild-type animals, we found that ARF causes kidney IL-18 levels to more than double and induces the conversion of the IL-18 precursor to the mature form. This conversion is not observed in caspase-1–/– ARF mice or sham-operated controls. We then injected wild-type mice with IL-18–neutralizing antiserum before the ischemic insult and found a similar degree of protection from ARF as seen in caspase-1–/– mice. In addition, we observed a fivefold increase in myeloperoxidase activity in control mice with ARF, but no such increase in caspase-1–/– or IL-18 antiserum–treated mice. Finally, we confirmed histologically that caspase-1–/– mice show decreased neutrophil infiltration, indicating that the deleterious role of IL-18 in ischemic ARF may be due to increased neutrophil infiltration.
Introduction
In the kidney, activation of caspases has been described during hypoxic proximal tubular necrotic injury in vitro and pan-caspase inhibition protects against this injury (1, 2). A recent study demonstrates that a pan-caspase inhibitor protects against ischemic ARF in mice by inhibition of distal tubule apoptosis and subsequent inflammation (3). In this study, however, the effect of caspase inhibition on the development of ischemic acute tubular necrosis (ATN), a well-established mechanism of tubular injury, was not studied. Also, in these studies, the use of pan-caspase inhibitors makes it difficult to implicate a specific caspase in hypoxic/ischemic injury. Thus, the mechanism of caspase-mediated ischemic ARF and the specific caspase involved in this renal ischemia-reperfusion injury is not clear.
The caspases are a family of intracellular cysteine proteases. Caspases participate in two distinct signaling pathways: (a) activation of proinflammatory cytokines and (b) promotion of apoptotic cell death (4). Caspase-1 (previously known as IL-1β–converting enzyme or ICE) plays a major role in the cleavage of the IL-1β precursor and the IL-18 precursor. Caspase-1 is remarkably specific for these precursors of IL-1β and IL-18 (IFN-γ–inducing factor) by making a single initial cut in each procytokine, which results in an active mature cytokine secreted into the extracellular space (5, 6). Although thymocytes from caspase-1–/– mice were found to be resistant to apoptosis induced by Fas Ab (7), subsequent studies did not demonstrate a role of caspase-1 in apoptosis (8).
To establish a pathogenic role of caspase-1 in cell injury, caspase-1–/– mice have been used. These caspase-1–/– mice have a defect in production of mature IL-1β and IL-18 and are protected against lethal endotoxemia (7, 8). The fact that IL-1β–/– mice are not protected against endotoxemia (9) suggests a potential role of IL-18 in the lethal outcome during sepsis. Moreover, in ischemic ARF, IL-1 receptor–knockout mice or mice treated with IL-1–receptor antagonist (IL-1Ra) are not protected against ischemic ARF (10). Taken together, therefore, these previous studies suggest that IL-18 may be a potential mediator of ischemic ARF. Thus, any protective effect against renal ischemia-reperfusion injury in caspase–/– mice may be due to a failure of this caspase to activate IL-18.
In the present study, we used caspase-1–/– mice to test the hypothesis that caspase-1 is a mediator of ischemic ARF in mice. The fact that expression of the proinflammatory cytokines IL-1β and IL-18 is altered in caspase-1–/– mice makes these mice a very suitable model for further studying the mechanism of ischemic ARF. Thus, the aims of the present study were to determine whether caspase-1–/– mice are protected against ischemic ARF and to explore the mechanisms of this protection, particularly the role of IL-18.
Methods
Animals.
Caspase-1–/– mice were generously provided by Richard A. Flavell, Yale University School of Medicine, New Haven, Connecticut, USA (7). These caspase-1–/– mice have been well characterized. They do not produce mature IL-1β after stimulation with lipopolysaccharide (LPS) (7), and splenocytes from these mice have defective release of active IL-18 (11). The same background male mice (B6/129-jF2) served as wild-type controls (The Jackson Laboratories, Bar Harbor, Maine, USA).
Ischemia protocol.
Mice weighing 20–25 g were anesthetized with an intraperitoneal injection of Avertin (2,2,2-tribromoethanol; Sigma-Aldrich, Milwaukee, Wisconsin, USA). A midline incision was made and the renal pedicles were bilaterally clamped for 22 minutes with microaneurysm clamps. The time of ischemia was chosen to obtain a reversible model of ischemic ARF and to avoid animal mortality. Serum creatinine reaches a peak at 24–48 hours of reperfusion and then gradually returns to normal within 5–7 days (data not shown). After 22 minutes, the clamps were removed. The kidneys were observed for restoration of blood flow returning to their original color. The abdomen was closed in two layers. Sham surgery consisted of the same surgical procedure except that clamps were not applied. During the 24-hour reperfusion period, the animals were kept in an incubator at 29°C. Blood samples were obtained by cardiac puncture at 24 hours after renal reperfusion or sham surgery. BUN and serum creatinine were measured using an Astra Autoanalyzer (Beckman Instruments Inc., Fullerton, California, USA).
Histological examination.
Paraformaldehyde-fixed (4%) and paraffin-embedded kidneys were sectioned at 4 μm and stained with hematoxylin-eosin and periodic acid–Schiff (PAS) using standard methods. Histological examinations were performed by the renal pathologist (M.S. Lucia) in a blinded fashion. Histological changes due to tubular necrosis, were quantitated by counting the percent of tubules that displayed cell necrosis, loss of brush border, cast formation, and tubule dilatation as follows: 0 = none, 1 = <10%, 2 = 11–25%, 3 = 26–45%, 4 = 46–75%, and 5 = >76%. At least 5–10 fields (×200) were reviewed for each slide.
Neutrophil infiltration was quantitatively assessed by the renal pathologist (M.S. Lucia) in a blinded fashion by counting the number of neutrophils per square millimeter at ×200 using a calibrated ocular grid. Five to ten fields were counted in the outer medulla on hematoxylin-eosin–stained slides.
Caspase-1 assay.
The activity of caspase-1 was determined by use of fluorescent substrates as we have described previously (2, 12), with modifications. Briefly, renal cortex was mixed with a lysis buffer containing 25 mM Na HEPES, 2 mM DTT, 1 mM EDTA, 0.1% 3-((3-cholamidopropyl) dimethylammonio)-1-pro-panesulfonate (CHAPS), 10% sucrose, 1 mM PMSF, and 1 μM pepstatin A, pH 7.2, and homogenized with ten strokes in a glass-Teflon homogenizer. The lysate was then centrifuged at 4°C at 100,000 g in a Beckman Ti70 rotor for 1 hour. The resultant supernatants were immediately frozen in liquid N2 and stored at –70°C until used. Lysate protein was measured by the Bradford method as described in the Bio-Rad protein assay kit with BSA as standards (Bio-Rad Laboratories Inc., Hercules, California, USA).
The caspase assay was performed as follows: 90 μl of extract (200–400 μg protein) and 10 μl of the substrate (final concentration, 50 μM) were added to 100 μl caspase assay buffer. The assay buffer contained 25 mM K+ HEPES, 1 mM DTT, 1 mM EDTA, 0.1% CHAPS, 1 mM DTT, sucrose 10%, pH 7.4. Acetyl-Tyr-Val-Ala-Asp-7-amido-4-methyl coumarin (Ac-YVAD-AMC) in 10% DMSO was used as a substrate for caspase-1–like proteases. The reaction was then initiated by addition of substrate. Peptide cleavage was measured over 1 hour at 30°C using a Cytofluor 4000 series fluorescent plate reader (PerSeptive Biosystems, Framingham, Massachusetts, USA) at an excitation wavelength of 380 nm and an emission wavelength of 460 nm. An AMC standard curve was determined for each experiment. Caspase activity was expressed in nanomoles of AMC released per minute of incubation time per milligram of lysate protein.
Western blot analysis.
Kidney cortices were homogenized in radioimmunoprecipitation assay (RIPA) buffer, and Western blot analysis was performed using standard protocols as described previously in detail (12). A mouse anti–caspase-1 mAb (1:1000; BD PharMingen, San Diego, California, USA) or a goat anti–IL-18 polyclonal Ab (1:100; Santa Cruz Biotechnology Inc., Santa Cruz, California, USA) were used.
In situ detection of DNA fragmentation.
The terminal deoxynucleotidyl transferase (TdT) mediated nick-end labeling (TUNEL) method was used to detect in situ DNA strand breaks. TACS 2 TdT-blue label in situ apoptosis detection kit (Trevigen Inc., Gaithersburg, Maryland, USA) was used. Paraffin sections were deparaffinized, rehydrated, incubated with proteinase K, and endogenous peroxidase activity was quenched. The sections were then treated with biotinylated nucleotide, manganese cation, and TdT enzyme. After treating with streptavidin-horseradish peroxidase (HRP), the sections were stained with blue label. The tissues were counterstained with nuclear fast red. TUNEL-positive cells in tubules were counted in ten random areas each in the cortex and medulla by a blinded observer.
Electrochemiluminescence assay for IL-18.
The electrochemiluminescence (ECL) assay for IL-18 in whole-kidney homogenates was performed as described previously in detail (11). The ECL assay detects both pro–IL-18 and mature IL-18.
Rabbit anti-murine IL-18 neutralizing antiserum.
Rabbit anti-murine IL-18 neutralizing antiserum was obtained from a New Zealand rabbit immunized by intradermal injection of murine recombinant IL-18 in the presence of Hunter’s titermax adjuvant (6). The IL-18 antiserum has been used in mice in vivo to block endogenous IL-18 (11, 13).
In vitro experiment to demonstrate caspase-1 cleavage of IL-18 in mouse kidney.
Cytosolic extracts were prepared from normal wild-type mouse kidney as described in the caspase-1 assay. Cytosolic extract (100 μg protein) was added to the caspase assay buffer without the DTT. The solution was incubated for 15 minutes at 37°C with either purified caspase-1 (obtained from Nancy Thornberry, Merck Research Laboratories, Rahway, New Jersey, USA) or purified caspase-3 (Upstate Biotechnology Inc., Lake Placid, New York, USA) in the presence or absence of the pan-caspase inhibitor Z-Val-Ala-Asp(Ome)-fluoromethyl ketone (Z-VAD-FMK) (400 μM). At the end of the experiment, samples were mixed with 5× sample buffer and stored at –20°C.
MPO assay.
MPO activity (kinetic assay) was measured as described previously with modifications (14). The snap-frozen kidney samples were homogenized in 3 ml of 20 mM potassium phosphate buffer, pH 7.4, and then centrifuged for 20 minutes at 40,000 g at 4°C. The pellet was resuspended in 3 ml hexadecyltrimethylammonium bromide (HDTB) buffer (13.7 mM of HDTB, 50 mM potassium phosphate, pH 6.0) (Sigma Chemical Co.). After three freeze-and-thaw cycles with sonication between cycles for 90 seconds (Vibracell Sonicator; Sonics & Materials Inc., Danbury, Connecticut, USA), the samples were placed in water bath at 60°C for 2 hours. Finally, the samples were again centrifuged at 16,400 g for 10 minutes and supernatant was decanted. Protein concentration was quantitated using Lowry assay. Twenty-five microliters of the supernatant was added to 725 μl of reaction buffer (0.68 mM O-dianisidine, 50 mM potassium phosphate buffer, pH 6.0, 29 mM H2O2). The change in absorbance was measured at 460 nm using a spectrophotometer (Beckman DU640; Beckman Instruments Inc.). MPO activity was measured during the first 2 minutes, calculated, and expressed in OD per minute per milligram of protein.
Immunohistochemistry.
Immunohistochemical localization was performed on paraformaldehyde-fixed and paraffin-embedded tissues using the avidin-biotin immunoperoxidase method. Paraffin sections were deparaffinized, rehydrated, endogenous peroxidase activity was quenched, and the sections were blocked with 3% donkey serum in PBS for 1 hour. The sections were then incubated with a goat polyclonal anti–IL-18 Ab (1:50 dilution; Santa Cruz Biotechnology Inc.). After incubating with biotinylated secondary Ab (Santa Cruz Biotechnology Inc.), tissues were treated with avidin-biotin-peroxidase complex (Santa Cruz Biotechnology Inc.). The Ab-bound peroxidase was visualized by 3,3′-diaminobenzidine (DAB) chromogen. Tissue sections were counterstained with methyl green.
Statistical analysis.
Non-normally distributed data were analyzed by the nonparametric unpaired Mann-Whitney test. Multiple group comparisons were performed using ANOVA with posttest according to Newman-Keuls. A P value of less than 0.05 was considered statistically significant. Values are expressed as means ± SE.
Results
Caspase-1 protein expression and caspase-1 activity are increased in ischemic ARF.
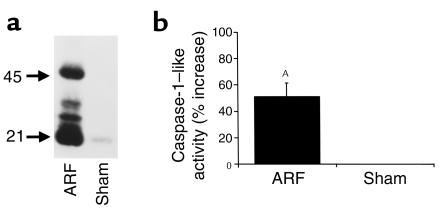
There was an increase in protein expression of both pro–caspase-1 (45 kDa) and active caspase-1 (21 kDa) in ischemic ARF compared with sham-operated controls (Figure 1a). To determine whether the increase in caspase-1 protein expression translates to increased activity, the activity of caspase-1–like protease was measured. There was a 51% increase in caspase-1–like activity in wild-type kidneys in ischemic ARF compared with sham-operated controls (Figure 1b).
Figure 1.
Caspase-1 protein expression (a) and activity (b) are increased in ischemic ARF. In wild-type mice, there was an increase in protein expression of both pro–caspase-1 (45 kDa) and active caspase-1 (21 kDa) in ischemic ARF compared with sham-operated controls (Sham). The immunoblot shown is representative of three separate experiments (a). Also, in wild-type mice, there was an increase in caspase-1–like activity in ischemic ARF compared with sham-operated controls (AP < 0.01 vs. sham, n = 7) (b).
Nitric oxide (NO) has emerged as a potent inhibitor of cysteine proteases. For example, in both in vitro and in vivo studies, NO prevents IL-1β and IL-18 release from macrophages by inhibiting caspase-1 (15). To determine whether NO regulates caspase-1 during ischemic ARF, we measured caspase-1 activity in sham-operated wild-type mice and iNOS-deficient mice with ARF. There was a 37% increase in caspase-1 activity in iNOS-deficient mice with ARF compared with sham-operated controls (P < 0.01, n = 5). If NO produced by iNOS during ischemic ARF was inhibiting caspase-1 activity, a greater increase in caspase-1 activity in iNOS-deficient mice with ARF, compared with wild-type mice with ARF, would have been expected. However, the increase in caspase-1 activity in iNOS-deficient mice with ARF did not exceed the 51% increase in caspase-1 activity in wild-type ARF (Figure 1b). These data do not support an interaction between NO and caspase-1 during ischemic ARF.
Caspase-1–/– mice are protected against ischemic ARF.
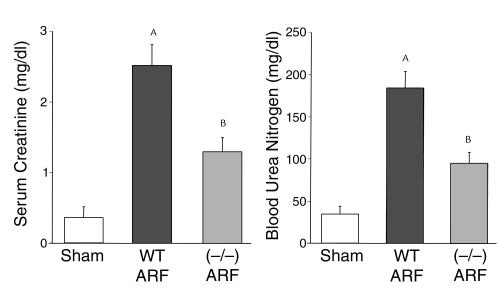
To determine whether the increase in caspase-1 activity plays a role in ischemic ARF, we used caspase-1–/– mice. Caspase-1–/– mice developed less severe ARF, as determined by BUN and serum creatinine, compared with wild-type mice (Figure 2).
Figure 2.
Caspase-1–/– mice are protected against ischemic ARF. Caspase-1–/– mice developed less severe ARF, as determined by BUN and serum creatinine, compared with sham-operated wild-type (WT) mice. AP < 0.001 vs. sham; BP < 0.01 vs. WT ARF; n = 8.
Apoptosis.
We next determined whether decreased apoptosis in the renal tubules of caspase-1–/– mice may be contributing to their protection against ischemic ARF. In situ DNA-strand breaks were quantitated using the TUNEL method during ischemic ARF. The presence of apoptotic cells was confirmed by detection of condensed, pyknotic nuclei on hematoxylin and eosin staining. The number of TUNEL-positive tubular cells per square millimeter in the cortex was 0.025 ± 0.025 in sham-operated kidneys, 1.4 ± 0.6 in wild-type ARF (not significant [NS] vs. sham) and 0.7 ± 0.4 in caspase-1–/– ARF kidneys (NS vs. wild-type ARF) (n = 4–10). The number of TUNEL-positive tubular cells per square millimeter in the medulla was 0.13 ± 0.13 in sham-operated kidneys, 4.7 ± 0.9 in wild-type ARF (P < 0.01 vs. sham), and 3.2 ± 1.2 in caspase-1–/– ARF kidneys (NS vs. wild-type ARF) (n = 4–10). Thus, while the number of TUNEL-positive cells in the medulla was increased in wild-type ARF compared with sham-operated controls, there was no difference in the cortex or medulla between wild-type and caspase-1–/– kidneys with ischemic ARF.
IL-18 protein.
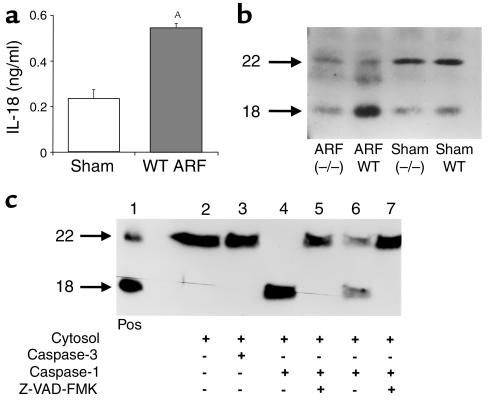
IL-18 concentration in kidney homogenates was increased in ischemic ARF in wild-type mice compared with sham-operated controls (Figure 3a). To differentiate the pro–IL-18 from the mature form, immunoblotting was performed using an Ab that recognizes both forms. In sham-operated wild-type and caspase-1–/– mice, IL-18 in whole kidney was predominantly present in the precursor form (22 kDa). In wild-type ischemic ARF there was a conversion of the pro–IL-18 (22 kDa) to active (18 kDa) IL-18. This conversion of the precursor to active form was attenuated in caspase-1–/– mice (Figure 3b).
Figure 3.
IL-18 in ischemic ARF. (a) IL-18 protein, measured by the ECL assay, was increased in ischemic ARF in wild-type (WT) mice compared with sham-operated controls (sham). AP < 0.01 vs. sham, n = 6. (b) In sham-operated wild-type (WT) and caspase-1–/– mice, IL-18 was predominantly in the pro–IL-18 form (22 kDa). In wild-type mice with ischemic ARF (ARF WT), there was a conversion of the pro–IL-18 form (22 kDa) to the active form (18 kDa). This conversion of the pro–IL-18 to active IL-18 form was attenuated in caspase-1–/– mice with ischemic ARF (ARF–/–). The immunoblot shown is representative of three separate experiments. (c) In an in vitro experiment, cytosolic extracts from normal wild-type WT mouse kidney were incubated with purified caspases. Recombinant murine pro–IL-18 (22 kDa) and active IL-18 (18 kDa) were used as a positive control (Pos) (lane 1). In the cytosolic extract with no additions, IL-18 was present only in the precursor form (lane 2) and addition of purified caspase-3 (10 ng) had no effect (lane 3). Addition of purified caspase-1 (10 ng) completely cleaved IL-18 from the precursor to active form (lane 4) and caspase-1 (1 ng) partially cleaved IL-18 (lane 6). Prior incubation with the caspase inhibitor, Z-VAD-FMK, prevents the cleavage of pro–IL-18 to active IL-18 (lanes 5 and 7). These data demonstrate that caspase-1, but not caspase-3, cleaves IL-18 in the mouse kidney.
To demonstrate that caspase-1 specifically cleaves IL-18 in the mouse kidney, cytosolic extracts were prepared from normal wild-type mouse kidney and incubated with either purified caspase-1 or purified caspase-3 (Figure 3c). Cytosolic extracts of normal mouse kidney contained pro–IL-18 that was converted to active IL-18 in the presence of purified caspase-1 but not caspase-3. The conversion of precursor to active IL-18 was prevented by addition of the caspase inhibitor Z-VAD-FMK.
Mice treated with rabbit anti-murine IL-18–neutralizing antiserum developed less severe ischemic ARF.
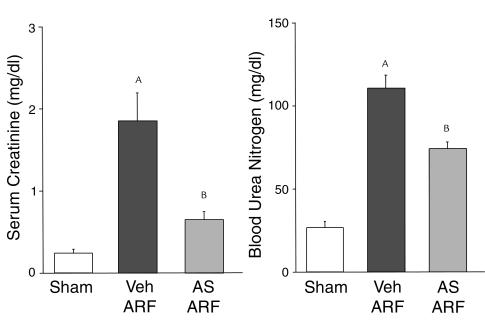
Rabbit anti-murine IL-18–neutralizing antiserum or vehicle (normal rabbit serum) was administered as follows: 300 μL intraperitoneally 40 minutes before renal pedicle clamp and 100 μL intraperitoneally just before clamp release. Wild-type mice treated with anti–IL-18 antiserum developed less severe ischemic ARF compared with vehicle-treated mice (Figure 4).
Figure 4.
Mice treated with rabbit anti-murine IL-18–neutralizing antiserum are protected against ischemic ARF. Wild-type mice treated with anti–IL-18 antiserum (AS) developed less severe ischemic ARF compared with vehicle-treated (normal rabbit serum; Veh) mice. AP < 0.001 vs. sham, BP < 0.05 vs. ARF Veh; n = 6.
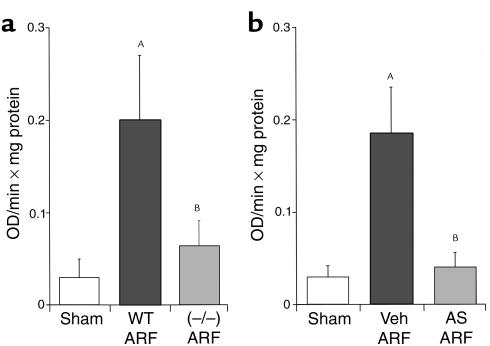
MPO activity and neutrophil infiltration.
MPO activity in ischemic ARF was decreased in caspase-1–/– mice (Figure 5a) and anti–IL-18 antiserum-treated mice (Figure 5b) compared with sham-operated wild-type mice. Thus, reduction of IL-18, either in caspase-1–/– mice or by administration of rabbit anti-murine IL-18–neutralizing antiserum to wild-type mice, results in a decrease in MPO activity.
Figure 5.
The increase in MPO activity during ischemic ARF is blocked in caspase-1–/– mice (a) and in mice treated with anti–IL-18 antiserum (b). In wild-type mice with ischemic ARF (WT ARF), there was an increase in MPO activity compared with sham-operated controls. In caspase-1–/– mice with ischemic ARF, MPO activity was normalized. AP < 0.001 vs. sham; BP < 0.01 vs. WT ARF; and NS vs. sham; n = 6 (a). In separate experiments, in wild-type mice treated with anti–IL-18 antiserum before ischemic ARF (AS ARF), MPO activity was normalized compared with vehicle-treated (Veh) animals. AP < 0.001 vs. sham; BP < 0.01 vs. Veh ARF; and NS vs. sham; n = 6 (b).
As MPO activity identifies monocytes/macrophages as well as neutrophils, neutrophil infiltration was quantified. Neutrophil infiltration (neutrophils per square millimeter) in the outer medulla was 4.3 ± 0.8 in sham-operated animals, 384 ± 46 in ischemia-reperfusion in wild-type mice (P < 0.01 vs. sham, n = 5), and 135 ± 59 in ischemia-reperfusion in caspase-1–/– mice (P < 0.01 vs. wild-type ischemia-reperfusion, n = 5).
Renal histopathology.
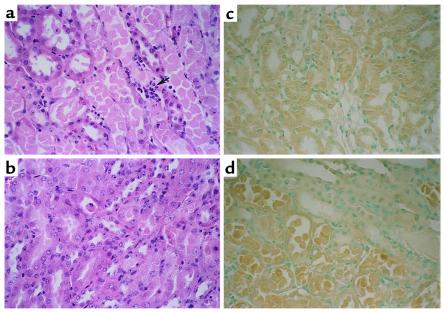
Histological scoring of ATN in the outer medulla was 0 in sham-operated animals, 4.8 ± 0.2 in ischemia-reperfusion in wild-type mice (P < 0.001 vs. sham, n = 5), and 3.2 ± 0.8 in ischemia-reperfusion in caspase-1–/– mice (P < 0.05 vs. wild-type ischemia-reperfusion, n = 5) (Figure 6, a and b).
Figure 6.
Renal histopathology (representative picture of at least three experiments). In wild-type mice with ischemic ARF, proximal tubules in the outer medulla show extensive damage including epithelial cell sloughing with focal denudation and numerous neutrophils (arrow) (a). In comparable sections from caspase-1–/– mice, tubules are largely intact with only focal sloughing of tubular cytoplasm and minimal loss of brush border. Neutrophils are inconspicuous in this case (b). Immunohistochemistry for IL-18 showed that there was cytoplasmic immunoreactivity in proximal tubule epithelium in sham-operated wild-type mice (c). There was increased IL-18 staining in necrotic proximal tubule epithelium in ischemic ARF in wild-type mice (d).
Immunohistochemical localization of IL-18 was performed. IL-18 was localized primarily in proximal tubules in sham-operated wild-type mice (Figure 6c) and in ischemic ARF (Figure 6d). Control sections were prepared by using the IgG preparation of the host instead of the primary Ab. Control sections showed no staining.
Urinary IL-18.
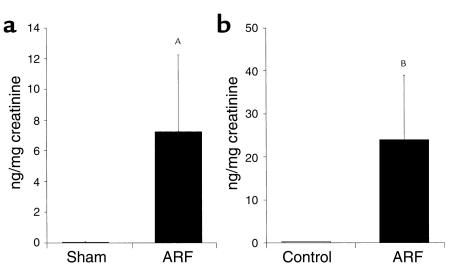
IL-18 was measured in mouse urine by the ECL method that detects both pro–IL-18 and mature IL-18. Urine was collected in metabolic cages from sham-operated and ischemic ARF mice for 24 hours after renal pedicle clamp. There was an increase in IL-18 in the urine of mice with ischemic ARF compared with virtually undetectable levels in sham-operated controls (Figure 7a).
Figure 7.
IL-18 was measured in the urine of mice with ARF by the ECL method that detects both the pro–IL-18 form and mature IL-18 form (a). IL-18 was also measured in the urine of human patients with ARF using a human IL-18 ELISA kit that detects the mature form of IL-18 (b). IL-18 levels were expressed per milligram of urinary creatinine to correct for differences in urine concentration. AP < 0.05 vs sham; BP = 0.01 vs controls; n = 4 for mouse and n = 5 for patients.
IL-18 was measured in human urine using a human IL-18 ELISA kit (Medical and Biological Laboratories, Nagoya, Japan) that specifically detects the mature form of IL-18 (16). The specificity of this kit for mature IL-18 was confirmed as follows: recombinant human pro–IL-18 (R & D Systems, Minneapolis, Minnesota, USA) was assayed using the human IL-18 ELISA kit. One nanogram per milliliter of pro–IL-18 was detected as 10 pg/ml of mature IL-18. Thus, the cross-reactivity of the kit for pro–IL-18 is extremely low. The patients with ARF all had an acute rise of serum creatinine to above 3 mg/dl and their urine sediment was characteristic of ATN, i.e., tubular cells and muddy brown broad granular casts. The cause of the ATN was renal ischemia caused by gastrointestinal hemorrhage (n = 2), severe congestive heart failure (n = 1), bone marrow transplantation (n = 1), and end-stage liver disease (n = 1). A total of five patients with ischemic ARF and five healthy controls were studied. The healthy patients had normal renal function and normal urinalysis as measured by dipstick and microscopy. The urine was centrifuged at 1,000 g for 5 minutes to remove sediment, and the assay was performed on the supernatant. Patients with ATN had increased mature IL-18 levels in the urine compared with virtually undetectable levels in normal controls (Figure 7b).
Discussion
The importance of caspases in tissue injury is evidenced by in vivo studies demonstrating that caspase inhibition protects against ischemic injury in brain (17), heart (18), and liver (19). During renal ischemia-reperfusion in vivo, there is increased caspase-3 activity (12). In freshly isolated proximal tubules, caspase inhibition protects against necrotic injury by inhibition of hypoxia-induced caspase activity (2). Also, inhibition of caspases protects against necrotic cell death induced by the mitochondrial inhibitor, antimycin A, in cultured proximal tubules (1). While the role of caspases in apoptotic cell death is well established, these studies provided novel evidence that caspase-1 may contribute to necrotic cell death as well. A recent study, however, demonstrates that the pan-caspase inhibitor, Z-VAD-FMK, provides functional protection against ischemic ARF by inhibition of apoptosis and subsequent inflammation (3). Thus, the specific caspase involved in ischemic ARF as well as the effect of caspase inhibition on acute tubular necrosis is not well established. Caspase-deficient mice are an excellent model to study the specific pathogenic role of individual caspases during ischemic ARF. Thus, we used caspase-1–/– mice to demonstrate the specific role of caspase-1 in ischemic ARF as well as to explore further the mechanism of tubular injury in ischemic ARF.
First we demonstrated that there is an increase in caspase-1 protein expression and activity during ischemic ARF and that caspase-1–/– mice are protected both functionally and histologically, against ischemic ARF. Caspase-1 is known to cleave both pro-IL1B and pro–IL-18 to their active forms (20, 21). Specifically, caspase-1, which cleaves pro–IL-1β, also cleaves pro–IL-18 at aspartic acid in the P1 position, producing a mature bioactive peptide that is readily released from the cell. Caspase-1–/– mice also have a defect in Fas-mediated apoptosis (7). Thus there are three possible mechanisms of this protection: (a) inhibition of IL-1β; (b) inhibition of IL-18, and (c) inhibition of apoptosis.
First we considered IL-1β as a mediator of the ischemic ARF. IL-1β is an important mediator of the inflammation that occurs during reperfusion injury (5). Thus, it is possible that IL-1β mediates the tubular damage. While there are other enzymes besides caspase-1 that cleave the IL-1β precursor into an active cytokine during local inflammatory processes (22), in ischemic ARF the role of caspase-1 activation would be to generate increased IL-1β. Previous studies, however, do not support such an effect. In cultured human proximal tubular cells exposed to hypoxia, there was an upregulation of ICAM-1 that was not associated with increased IL-1β (23). In hypoxia–induced injury in freshly isolated proximal tubules, the IL-1–receptor antagonist (IL-1Ra) exhibited no protective effect and recombinant IL-1β did not worsen the injury (2). Thus, IL-1β does not directly mediate hypoxia-induced proximal tubular injury. These in vitro results in proximal tubules are supported by in vivo studies. Rats treated with IL-1 before ischemia did not develop worse renal failure (24). In a model of ischemic ARF in mice, identical to that used in the present study, IL-1Ra (10 mg/kg) did not have a protective effect (10). Moreover, IL-1 receptor–knockout mice are not protected against ischemic ARF (10).
Next we considered inhibition of apoptosis as a mechanism of protection against ischemic ARF in caspase-1–/– mice. There was an increase in apoptotic tubular cells in the medulla in ischemic ARF compared with sham-operated controls. However, in the face of functional and histological (decreased tubular necrosis) protection against ischemic ARF in caspase-1–/– mice, there was a lack of inhibition of apoptosis. In fact, the role of caspase-1 in apoptosis is controversial because caspase-1–/– mice developed by another research group do not have a defect in apoptosis (8).
Finally, we considered IL-18 as a mediator of the ischemic ARF in caspase-1–/– mice. Evidence for a role of IL-18 as a mediator of tissue injury in other organs is emerging. Neutralization of IL-18 during lethal endotoxemia reduces neutrophil tissue accumulation and protects mice against the lethal effects of LPS (13). IL-18 has also been shown to be involved in lung injury after endotoxemia (25) as well as Shigella flexneri–induced inflammation (26). A possible role of IL-18 in ischemic ARF in mice is suggested by the observation of an upregulation of IL-18 mRNA that coincided with caspase-1 activation (27). To our knowledge, there have been no other studies of the role for IL-18 in ischemic organ injury. In the present study, the protection against ischemic ARF in the caspase-1–/– mice suggested a potential pathogenic role of IL-18. Furthermore, the concept that IL-18 may play an important pathogenic role in ischemic ARF was supported by the demonstration of increased IL-18 protein and a conversion of the pro–IL-18 form to the active form of IL-18 during ischemic ARF. Lastly, the administration of anti–IL-18 antiserum was shown to protect mice against ischemic ARF.
To determine a possible mechanism of IL-18–induced tissue injury, we measured MPO activity in the kidney. Neutralization of IL-18 was accompanied by a decrease of MPO content in the kidney, thus reflecting a reduction in leukocyte infiltration. While the role of neutrophils in ARF remains controversial, there is increasing evidence that leukocytes, particularly neutrophils, mediate tissue injury and play a role in the development of ARF (28). This experimental evidence derives from studies (28) that show an accumulation of neutrophils in ischemic ARF, neutrophil depletion studies that show a reduction of tissue injury, and studies demonstrating a beneficial role of anti–ICAM-1 therapy in ARF.
While IL-18 exerts some of its proinflammatory effects by induction of IFN-γ, recent data suggest that it also has direct anti-inflammatory effects. For example, IL-18 induces production of chemokines, such as IL-8 and macrophage inflammatory protein-1α (MIP-1α) (29), and upregulates expression of vascular cell adhesion molecule (VCAM-1) in hepatic melanoma cells in vivo (30). In this regard, upregulation of another chemokine, endothelial monocyte-activating polypeptide II (EMAP-II) as well as the adhesion molecule, ICAM-1, plays an important pathogenic role in ischemic ARF (31).
Constitutive levels of IL-18 mRNA and protein are present in unstimulated human and murine cells and in the tissues of healthy mice (11). In the present study, immunohistochemistry demonstrated the presence of IL-18 in tubular epithelial cells in normal sham-operated kidneys. The presence of IL-18 in tubular lumens on immunohistochemistry of ischemic ARF kidneys prompted us to measure IL-18 in the urine. The observed presence of IL-18 in the urine during ARF is compatible with the fact that caspase-1, an intracellular cysteine protease, cleaves pro–IL-18 into mature IL-18, a bioactive peptide that is readily released from the cell. The specificity and timing of the appearance of IL-18 in the urine in ARF deserves further study, particularly given the need for early diagnostic markers and interventions in this high-mortality clinical entity.
In conclusion, during ischemic ARF, caspase-1–mediated conversion of pro–IL-18 to active IL-18 occurs. The active IL-18 is released from the tubular cell and mediates neutrophil infiltration during ischemic ARF. Administration of neutralizing anti–IL-18 Ab’s affords protection against ischemic ARF. Thus, the present results demonstrate that IL-18 plays a deleterious role in experimental ischemic ARF, perhaps in part due to increasing neutrophil infiltration into the renal parenchyma. The detection of IL-18 in the urine and the potential therapeutic effect of neutralizing IL-18 may have future clinical implications for ARF.
Acknowledgments
This work was supported by the NIH grants DK-52599 and AI-15614 and a National Kidney Foundation Young Investigator Grant.
References
- 1.Kaushal GP, Ueda N, Shah SV. Role of caspases (ICE/CED 3 proteases) in DNA damage and cell death in response to a mitochondrial inhibitor, antimycin A. Kidney Int. 1997;52:438–445. doi: 10.1038/ki.1997.350. [DOI] [PubMed] [Google Scholar]
- 2.Edelstein CL, Shi Y, Schrier RW. Role of caspases in hypoxia-induced necrosis of rat renal proximal tubules. J Am Soc Nephrol. 1999;10:1940–1949. doi: 10.1681/ASN.V1091940. [DOI] [PubMed] [Google Scholar]
- 3.Daemen MARC, et al. Inhibition of apoptosis induced by ischemia-reperfusion prevents inflammation. J Clin Invest. 1999;104:541–549. doi: 10.1172/JCI6974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fraser A, Evan G. A license to kill. Cell. 1996;85:781–784. doi: 10.1016/s0092-8674(00)81005-3. [DOI] [PubMed] [Google Scholar]
- 5.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 6.Fantuzzi G, Puren AJ, Harding MW, Livingston DJ, Dinarello CA. Interleukin-18 regulation of interferon gamma production and cell proliferation as shown in interleukin-1 beta-converting enzyme (caspase-1)-deficient mice. Blood. 1998;91:2118–2125. [PubMed] [Google Scholar]
- 7.Kuida K, et al. Altered cytokine export and apoptosis in mice deficient in interleukin-1B converting enzyme. Science. 1995;267:2000–2002. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- 8.Li P, et al. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell. 1995;80:401–411. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- 9.Fantuzzi G, et al. Effect of endotoxin in IL-1 beta-deficient mice. J Immunol. 1996;157:291–296. [PubMed] [Google Scholar]
- 10.Haq M, Norman J, Saba SR, Ramirez G, Rabb H. Role of IL-1 in renal ischemic reperfusion injury. J Am Soc Nephrol. 1998;9:614–619. doi: 10.1681/ASN.V94614. [DOI] [PubMed] [Google Scholar]
- 11.Fantuzzi G, Reed DA, Dinarello CA. IL-12-induced IFN-gamma is dependent on caspase-1 processing of the IL-18 precursor. J Clin Invest. 1999;104:761–767. doi: 10.1172/JCI7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi Y, Melnikov VY, Schrier RW, Edelstein CL. Down-regulation of the calpain inhibitor protein, calpastatin, by caspases during renal ischemia. Am J Physiol . 2000;279:F509–F517. doi: 10.1152/ajprenal.2000.279.3.F509. [DOI] [PubMed] [Google Scholar]
- 13.Netea MG, et al. Neutralization of IL-18 reduces neutrophil tissue accumulation and protects mice against lethal Escherichia coli and Salmonella typhimurium endotoxemia. J Immunol. 2000;164:2644–2649. doi: 10.4049/jimmunol.164.5.2644. [DOI] [PubMed] [Google Scholar]
- 14.Hillegass LM, Griswold DE, Brickson B, Albrightson-Winslow C. Assessment of myeloperoxidase activity in whole rat kidney. J Pharmacol Methods. 1990;24:285–295. doi: 10.1016/0160-5402(90)90013-b. [DOI] [PubMed] [Google Scholar]
- 15.Kim YM, Talanian RV, Li J, Billiar TR. Nitric oxide prevents IL-1beta and IFN-gamma-inducing factor (IL-18) release from macrophages by inhibiting caspase-1 (IL-1beta-converting enzyme) J Immunol. 1998;161:4122–4128. [PubMed] [Google Scholar]
- 16.Shibata M, et al. Increased concentrations of plasma IL-18 in patients with hepatic dysfunction after hepatectomy. Cytokine. 2000;12:1526–1530. doi: 10.1006/cyto.2000.0740. [DOI] [PubMed] [Google Scholar]
- 17.Schielke GP, Yang GY, Shivers BD, Betz AL. Reduced ischemic brain injury in interleukin-1 beta converting enzyme-deficient mice. J Cereb Blood Flow Metab. 1998;18:180–185. doi: 10.1097/00004647-199802000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Yaoita H, Ogawa K, Maehara K, Maruyama Y. Attenuation of ischemia/reperfusion injury in rats by a caspase inhibitor. Circulation. 1998;97:276–281. doi: 10.1161/01.cir.97.3.276. [DOI] [PubMed] [Google Scholar]
- 19.Natori S, et al. Apoptosis of sinusoidal endothelial cells occurs during liver preservation injury by a caspase-dependent mechanism. Transplantation. 1999;68:89–96. doi: 10.1097/00007890-199907150-00018. [DOI] [PubMed] [Google Scholar]
- 20.Gu Y, et al. Activation of interferon-gamma inducing factor mediated by interleukin-1beta converting enzyme. Science. 1997;275:206–209. doi: 10.1126/science.275.5297.206. [DOI] [PubMed] [Google Scholar]
- 21.Ghayur T, et al. Caspase-1 processes IFN-gamma-inducing factor and regulates LPS-induced IFN-gamma production. Nature. 1997;386:619–623. doi: 10.1038/386619a0. [DOI] [PubMed] [Google Scholar]
- 22.Coeshott C, et al. Converting enzyme-independent release of tumor necrosis factor alpha and IL-1beta from a stimulated human monocytic cell line in the presence of activated neutrophils or purified proteinase 3. Proc Natl Acad Sci USA. 1999;96:6261–6266. doi: 10.1073/pnas.96.11.6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Combe C, et al. Hypoxia induces intercellular adhesion molecule-1 on cultured human tubular cells. Kidney Int. 1997;51:1703–1709. doi: 10.1038/ki.1997.235. [DOI] [PubMed] [Google Scholar]
- 24.Guidot DM, et al. Interleukin-1 treatment increases neutrophils but not antioxidant enzyme activity or resistance to ischemia-reperfusion injury in rat kidneys. Inflammation. 1994;18:537–545. doi: 10.1007/BF01560700. [DOI] [PubMed] [Google Scholar]
- 25.Arndt PG, Fantuzzi G, Abraham E. Expression of interleukin-18 in the lung after endotoxemia or hemorrhage-induced acute lung injury. Am J Respir Cell Mol Biol. 2000;22:708–713. doi: 10.1165/ajrcmb.22.6.3832. [DOI] [PubMed] [Google Scholar]
- 26.Sansonetti PJ, et al. Caspase-1 activation of IL-1beta and IL-18 are essential for Shigella flexneri-induced inflammation. Immunity. 2000;12:581–590. doi: 10.1016/s1074-7613(00)80209-5. [DOI] [PubMed] [Google Scholar]
- 27.Daemen MA, Van’t Veer C, Wolfs TG, Buurman WA. Ischemia-reperfusion-induced IFN-gamma up-regulation: involvement of IL-12 and Il-18. J Immunol. 1999;162:5506–5510. [PubMed] [Google Scholar]
- 28.Heinzelmann M, Mercer-Jones MA, Passmore JC. Neutrophils and renal failure. Am J Kidney Dis. 1999;34:384–399. doi: 10.1016/s0272-6386(99)70375-6. [DOI] [PubMed] [Google Scholar]
- 29.Puren AJ, Fantuzzi G, Gu Y, Su MS, Dinarello CA. Interleukin-18 (IFNgamma-inducing factor) induces IL-8 and IL-1beta via TNFalpha production from non-CD14+ human blood mononuclear cells. J Clin Invest. 1998;101:711–721. doi: 10.1172/JCI1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vidal-Vanaclocha F, et al. IL-18 regulates IL-1beta-dependent hepatic melanoma metastasis via vascular cell adhesion molecule-1. Proc Natl Acad Sci USA. 2000;97:734–739. doi: 10.1073/pnas.97.2.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelly KJ, Williams WW, Jr, Colvin RB, Bonventre JV. Intracellular adhesion molecule-1 deficient mice are protected against ischemic renal injury. J Clin Invest. 1996;97:1056–1063. doi: 10.1172/JCI118498. [DOI] [PMC free article] [PubMed] [Google Scholar]