Figure 1.
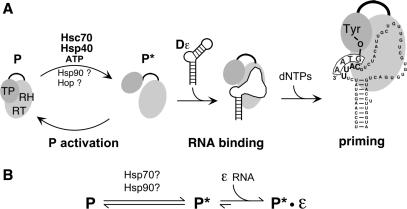
(A) Current model of replication initiation by hepadnaviral P proteins. DHBV P protein, with its terminal protein (TP) and reverse transcriptase/RNase H (RT/RH) domains linked through a dispensable spacer, is unable to bind Dε RNA without prior chaperone-mediated conversion into a metastable, active conformation (P*). In vitro activation strictly requires Hsc70, Hsp40 and ATP. The necessity for Hop and Hsp90 has been controversial (31,33). Dε RNA binding is accompanied by structural changes in the RNA and the RT, enabling the synthesis of a short DNA oligonucleotide templated by a bulged region within Dε (priming); its 5′-terminal nucleotide is covalently linked to a Tyr residue in the TP domain. (B) Different states of hepadnaviral RT activation. P represents the non-activated state. Chaperones and energy are required to produce, and maintain, activated metastable P* which is able to bind Dε RNA. The steady-state P* concentration depends on the rates of P* production and decay. Upon energy depletion P* decays within minutes whereas P–Dε RNA complexes are stable over hours (31).