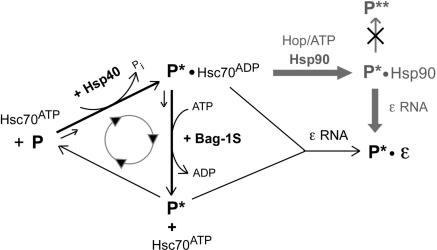
Figure 8.
Model for chaperone contributions to P protein activation. As in the general Hsp70 chaperoning cycle, Hsp40 stimulates the ATPase activity of Hsc70, converting Hsc70ATP into high substrate affinity Hsc70ADP which binds P and produces P*-Hsc70ADP. Spontaneous recycling of P*-bound Hsc70ADP into free Hsc70ATP is rate limiting. Proper concentrations of Bag-1S promote ATP for ADP exchange on Hsc70 and substrate release, enabling faster cycling and faster production of P*, increasing its steady-state concentration. Over-acceleration by excess Bag-1S reduces the time span Hsc70ADP can act on P, reducing P to P* conversion and thus the steady-state P* concentration. Dε RNA may react with either free P* or P*-Hsc70ADP. While the increased release of P* by Bag-1S would favor free P* as binding partner, the simultaneously generated Hsc70ATP becomes available for a new round of P*-Hsc70ADP production. The cyclic nature of this process thus prevents a clear distinction between the two options. Hsp90 (pathway in gray) does not act directly on P but requires prior Hsc70 action; the Hop dependence strongly suggests the P*–Hsc70ADP complex, not free P*, as Hsp90 substrate. Hsp90 increases the steady-state levels of P*, by slowing down decay or promoting production, but does not induce formation of a more active form P** (crossed-out pathway). The exact compositions of the chaperone-containing complexes are not known and may be subject to dynamic changes. Similarly, not each single interaction between Hsc70 and P may be productive in P* generation.