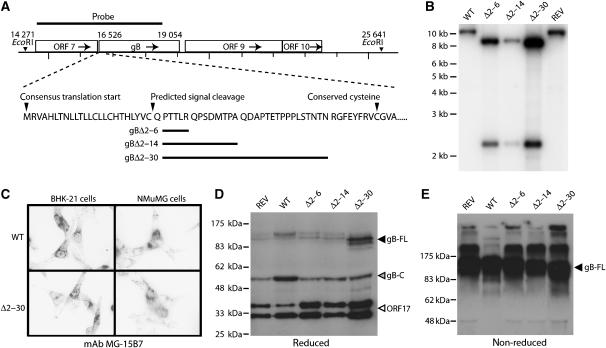
Figure 1.
Generation of MuHV-4 mutants lacking gB-NT. (A) The indicated regions of the gB coding sequence were removed and replaced with an EcoRI site. (B) Southern blotting showed the predicted introduction of an EcoRI restriction site for each deletion mutant (gBΔ2–6, gBΔ2–14, gBΔ2–30). REV, revertant of the gBΔ2–30 mutant. (C) Immunofluorescence of cells infected with the largest deletion mutant (gBΔ2–30) showed no effect on gB expression in either BHK-21 fibroblasts or NMuMG epithelial cells. gB-specific staining is black in this reversed image. Similar staining was observed with the gBΔ2–6 and gBΔ2–14 mutants. (D) Immunoblot of wild-type (WT), gBΔ2–6, gBΔ2–14 and gBΔ2–30 virions with the gB-C-specific mAb MG-4D11. REV, revertant of the 2–30 deletion mutant; gB-FL, uncleaved gB; gB-C, C-terminal half of furin-cleaved gB (Lopes et al, 2004), which contains the MG-4D11 epitope. The ORF17-specific mAb 150-7D1 was included in the same blot to give a loading control. The two ORF17 bands correspond to a post-translational cleavage, as described for the homologous herpes simplex virus UL26. (E) Unreduced gB immunoblotted with mAb MG-4D11 shows a single major band (gB-FL) since the furin-cleaved gB fragments are held together by disulfide bonds. Higher molecular weight forms are presumably gB multimers or aggregates.