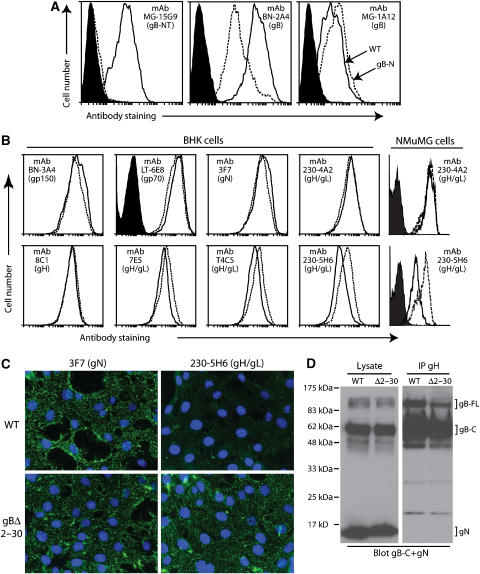
Figure 3.
Antigenicity of the gBΔ2–30 fusion complex. (A) BHK-21 cells were infected (2 p.f.u./cell, 18 h) with wild-type (solid lines) or gBΔ2–30 mutant (dotted lines) MuHV-4, and then analyzed by flow cytometry. The filled histogram shows uninfected cells. MAb 15G9 recognizes gB-NT, mAb BN-2A4 recognizes an epitope elsewhere in the N-terminal half of gB and mAb MG-1A12 recognizes a complex gB epitope. (B) BHK-21 or NMuMG cells were infected as for (A) and analyzed by flow cytometry for the expression of other MuHV-4 glycoproteins. Again, the dotted line corresponds to the gBΔ2–30 mutant and the solid line corresponds to wild type. The filled histogram shows secondary antibody only staining. Each histogram shows 30 000 events. The reduction in gH/gL staining for T4C5, 7E5 and 230-5H6 were all statistically significant (P<0.001 by Student's t-test). Equivalent data were obtained in four further experiments. (C) NMuMG cells were exposed to wild-type (WT) or gBΔ2–30 virions (2 h, 4°C), then washed with PBS and fixed with 2% paraformaldehyde. The cells were then stained for gN with mAb 3F7 and for gH/gL with mAb 230-5H6. Nuclei were counter-stained with DAPI. (D) Wild-type (WT) and gBΔ2–30 virions were lysed in 1% digitonin. gH was immunoprecipitated with mAb 8C1 (IgG2b). The original lysates and the precipitates (IP gH) were then immunoblotted for gB-C with mAb MG-4D11 and for gN with mAb 3F7 (both IgG2a) May et al (2005). Detection was with an IgG2a-specific secondary antibody. The positions of gN and full-length (gB-FL) and cleaved (gB-C) gB are marked. While both gB and gN were present in the lysates, only gB was co-precipitated with gH. No difference in co-precipitate was observed between the gBΔ2–30 mutant and wild type. Equivalent data were obtained in two further experiments.