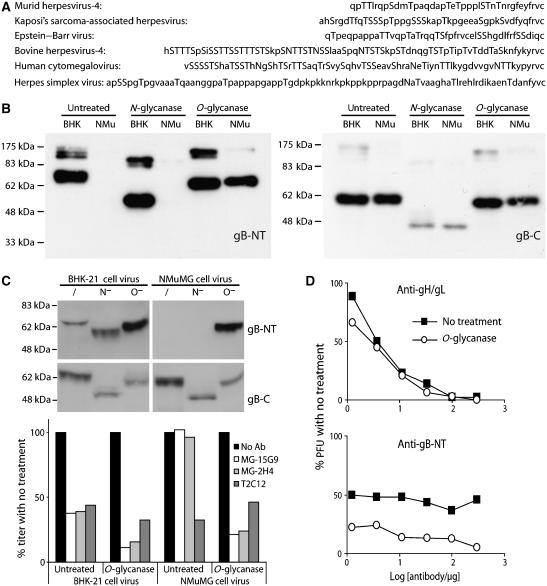
Figure 6.
O-linked glycans mask gB-NT of NMuMG cell-derived virions. (A) Herpesvirus gB alignment from the predicted signal sequence cleavage site to the first conserved cysteine residue, with potential glycosylation sites, both N- and O-linked, in upper case. (B) Reduced MuHV-4 virion lysates derived from BHK-21 (BHK) or NMuMG cells (NMu) were either left untreated or deglycosylated as indicated, then immunoblotted for gB-NT with mAb MG-2C10 or for gB-C with mAb MG-4D11. (C) Intact BHK-21 or NMuMG cell-derived virions were deglycosylated without denaturation. /, no enzyme, N−, protein N-glycanase, O−, sialidase A+O-glycanase. Each was then immunoblotted for gB-NT/gB-C and tested for neutralization. MAbs MG-15G9 and MG-2H4 are gB-NT-specific, T2C12 is gH/gL-specific. Equivalent data were obtained in two further experiments. Comparing results for all the experiments by Student's t-test, T2C12 neutralization was not significantly different between untreated and deglycosylated samples, whereas MG-15G9 and MG-2H4 showed a significant improvement in neutralization with deglycosylation for both BHK-21 cells (P<0.02) and NMuMG cells (P<0.001). (D) MuHV-4 virions were recovered from infected mouse lungs, deglycosylated (O-glycanase) or not (no treatment) as in (C), then exposed to gH/gL-specific (T2C12) or gB-NT-specific (MG-2C10)-neutralizing mAbs. The improvement in gB-NT-directed neutralization by deglycosylation was significant by χ2 test (P<0.002). Equivalent data were obtained in a repeat experiment.