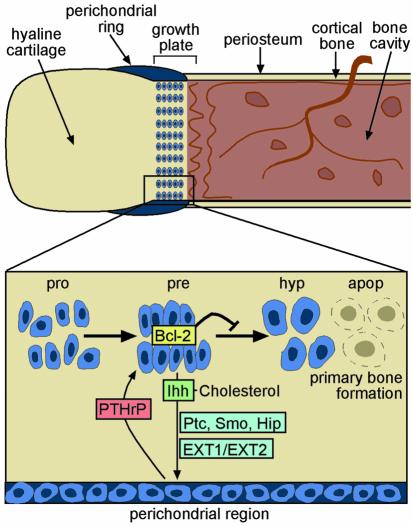
Figure 2.
A working model of mammalian bone development. Hypothetical representation of the growth plate during endochondral bone formation (adapted from ref. 44). Prehypertrophic chondrocytes (pre) localized within the growth plate produce a cell signaling molecule, Ihh, which diffuses to the receiving cells via HS proteoglycans (HSPGs) that are glycosylated by EXT1 and EXT2 HS-Pol activity. By analogy with what is known about the Hh signaling cascade in Drosophila, it is likely that signaling is initiated when Ihh binds to a two-component receptor/signal transducer complex on the surface of a responding cell, which is composed of both Patched (Ptc) and Smoothened (Smo). When Ihh binds to Ptc, Smo is de-repressed and additional intracellular components of the signaling pathway are activated (reviewed in ref. 29). Recent work suggests that there may be an additional member in this cascade, Hedgehog-interacting protein (Hip), which could act to attenuate Ihh signaling. In bone, Ptc is expressed on the osteogenic cells of the perichondral region, and Ihh binding induces chondrocyte proliferation by upregulating a second signaling molecule, PTHrP. PTHrP binds to the PTH/PTHrP receptor on a subpopulation of proliferating (pro) and prehypertrophic chondrocytes, thereby inducing production of Bcl-2, a well known antiapoptotic protein. In the absence of negative feedback, chondrocytes differentiate into hypertrophic chondrocytes (hyp), which undergo apoptosis and are then replaced by bone-forming osteoblasts. According to this model, hereditary exostosis formation would occur when a chondrocyte bearing a germline mutation in either EXT1 or EXT2 develops an inactivating “hit” in the second copy of the same gene, thereby abrogating HS expression. Clonal expansion of this mutant chondrocyte would then lead to a local perturbation in Ihh diffusion with, as a result, release from negative feedback control. The neighboring population of proliferating chondrocytes would therefore undergo premature chondrocyte differentiation, apoptosis, and subsequent ossification.