Abstract
Background
Adolescence is a period of elevated alcohol consumption in humans as well as in animal models. Previous studies in our laboratory have shown that adolescent Sprague–Dawley rats consume approximately 2 times more ethanol on a gram per kilogram basis than adult animals in a 2-bottle choice free-access situation. The purpose of the present study was to examine the time course and pattern of elevated ethanol intake during adolescence and the adolescent-to-adult transition, contrast this intake with ontogenetic patterns of food and water intake, and determine whether adolescent access to ethanol elevates voluntary consumption of ethanol in adulthood.
Methods
Adolescent [postnatal day (P)27–28] and adult (P69–70) male Sprague–Dawley rats were singly housed with continuous access to both water and 1 of 3 experimental solutions in ball-bearing–containing sipper tubes: unsweetened ethanol (10% v/v), sweetened ethanol (10% v/v+0.1% w/v saccharin), and saccharin alone (0.1% w/v).
Results
Ethanol consumption plateaued at approximately 7.5 g/kg/d during the first 2 weeks of measurement (i.e., P28–39) in early adolescence, before declining sharply at approximately P40 to levels that were only modestly elevated compared with adult-typical consumption patterns that were reached by approximately P70. In contrast, intake of food and total calories showed a more gradual decline into adulthood with no distinguishable plateaus in early adolescence. When adolescent-initiated and adult-initiated animals were tested at the same chronological age in adulthood, animals drank similar amounts regardless of the age at which they were first given voluntary access to ethanol.
Conclusions
Taken together, these data suggest that the elevated ethanol intake characteristic of early-to-mid adolescence is not simply a function of adolescent-typical hyperphagia or hyperdipsia, but instead may reflect age-related differences in neural substrates contributing to the rewarding or aversive effects of ethanol, as well as possible modulatory influences of ontogenetic differences in sensitivity to novelty or in ethanol pharmacokinetics. Voluntary home cage consumption of ethanol during adolescence, however, was not found to subsequently elevate ethanol drinking in adulthood.
Keywords: Adolescence, Rat, Ethanol, Free-Access, Consumption
The Transition through adolescence is a unique developmental period. It is characterized by numerous behavioral, hormonal, and neural changes in humans as well as nonhuman mammals (see Spear, 2000 for a review). Among the behaviors characteristic of both human adolescents (Arnett, 1992; Maggs et al., 1995) as well as adolescent rodents (Primus and Kellogg, 1989; Spear et al., 1980; Vanderschuren et al., 1997) are increases in risk-taking and peer-directed social interactions. Drug and alcohol experimentation also becomes common among human adolescents during this time. The prevalence of alcohol use and misuse is surprisingly high in this age group, with 80% of 12th graders reporting having tried alcohol at least once and almost 30% of them reporting binge consumption within the past 2 weeks (Johnston et al., 2001). This high rate of alcohol use may have implications for development of later alcohol use disorders. Although causality cannot be inferred from these data, the results from the National Longitudinal Alcohol Epidemiological Survey found that initiation of alcohol use before the age of 14 increased the rate of lifetime alcohol dependency to 40%, whereas the rate was only 10% in those initiating use after the age of 20 (Grant and Dawson, 1997).
As ethical constraints limit investigation of factors that contribute to elevated alcohol consumption during adolescence in humans, animal models of ethanol (EtOH) intake during adolescence are of importance. In a rat model, postnatal days (P)28 to 42 have been conservatively defined as prototypic adolescence based on characteristic neurobehavioral changes, with some signs of adolescence persisting in male adolescents until P55 to 60 (Spear, 2000).
This time period itself may not be homogeneous, and can be further subdivided functionally into early, mid, and late phases (see Adriani et al., 2002; Varlinskaya and Spear, 2004, 2006a). Support for distinct subphases within the broader adolescent period includes the literature describing differential behavioral sensitivity to ethanol and other drugs of abuse during early versus late adolescence (see Adriani et al., 2002; Belluzzi et al., 2004; Silveri and Spear, 1998; Varlinskaya and Spear, 2006a). For instance, when examining hypnotic effects following challenge with a high dose of ethanol, early adolescent Sprague–Dawley rats were found to recover more rapidly from ethanol-induced sedation than late adolescent animals (Silveri and Spear, 1998). Similarly, early adolescent Sprague–Dawley rats (P28) were found to be more sensitive to ethanol-induced social facilitation in a familiar environment and less sensitive to the anxiolytic effects of ethanol in an unfamiliar environment than their late adolescent (P42) counterparts (Varlinskaya and Spear, 2006a).
Although few studies using animal models have compared voluntary ethanol intake in adolescents and adults, several of the available studies have reported that elevated ethanol consumption is not limited to human adolescents (Doremus et al., 2005; Lancaster et al., 1996). In other studies, however, relatively moderate levels of voluntary ethanol intake have been reported in adolescent Long–Evans rats (Honey and Galef, 2003; Siciliano and Smith, 2001), although these studies were not designed to directly compare adolescent ethanol consumption with that of adults. Methodological differences across experiments may contribute to the mixed results observed in the literature. Previous research within our laboratory (Doremus et al., 2005) has repeatedly found that adolescent Sprague–Dawley rats voluntarily consume more ethanol than adults on a gram per kilogram basis, with adolescent intake tending to decline over the course of the 1 to 2-week assessment period while remaining significantly higher than that of adults. However, various environmental and procedural variables have been shown to exert notable influences on this intake (Doremus et al., 2005). For instance, in a home cage, 2-bottle choice paradigm, ethanol intake of adolescents was found to be insensitive to the intake-suppressing effect of single housing seen in adults, with adolescent animals drinking similar amounts of ethanol regardless of experimental housing condition (Doremus et al., 2005). Similarly, in a study examining postweaning housing conditions and initiation of ethanol-drinking procedure, McKinzie et al. (1998) found that neither postweaning housing condition nor initiation procedure affected levels of ethanol consumption in periadolescent alcohol-preferring (P) rats. In contrast, ethanol intake among adolescent, but not adult Sprague–Dawley rats, was found to be exacerbated when the solution was presented in ball-bearing–containing ball-point (BP) sipper tubes rather than open-ended tubes, with adolescents drinking from BP tubes developing a preference for ethanol over water (Doremus et al., 2005). Elevated ethanol intake among adolescents was also shown in this study not to be due to the inherent high caloric value of the ethanol solution.
Despite evidence that early alcohol exposure is correlated with later alcohol dependency and alcohol use disorders in humans (Dewit et al., 2000; Grant and Dawson, 1997), there have been relatively few studies in laboratory animals examining the impact of chronic adolescent ethanol exposure on later consumption in adulthood in outbred animals. Studies looking at ethanol exposure during adolescence and later ethanol drinking in adulthood have found mixed results. Male periadolescent BALB/cByJ mice given a 2-bottle choice access to ethanol were found to have increased ethanol preference in adulthood (Blizard et al., 2004). Studies examining free-choice ethanol exposure during adolescence and later operant self-administration of ethanol in adulthood in P rats have found that P rats exposed during adolescence learned to self-administer ethanol more rapidly in adulthood and displayed more ethanol-seeking behavior as adults than naïve P rats (Rodd-Henricks et al., 2002a). In a companion study, adult P rats given free-choice pre-exposure beginning in adulthood and later tested after a comparable amount of time did not show increased ethanol seeking in an operant paradigm (Rodd-Henricks et al., 2002b). In another study comparing 24-hour drinking patterns of adolescent and adult P rats over a 4-week period, adolescent male rats were found to exhibit more of an increase in ethanol licking behavior across weeks than adult males (Bell et al., 2006). High-alcohol–drinking rats (HAD) given 2-bottle choice access to ethanol solutions and water throughout adolescence and early adulthood (P30–60) also showed increased consumption (g/kg/d) and preference for ethanol over time (Bell et al., 2004). However, forced administration via ethanol vapor or forced consumption during adolescence did not result in increased intake in adult Sprague–Dawley rats (Slawecki and Betancourt, 2002; Tolliver and Samson, 1991). Most of these studies exposing animals to ethanol during adolescence assessed ethanol-drinking behavior in adulthood after a period of abstinence in postadolescence or early adulthood and did not include an adult comparison group or, alternatively, were conducted in animals bred to prefer alcohol.
The purpose of the present study was to examine the time course and pattern of elevated ethanol intake seen in adolescent animals and during the adolescent-to-adult transition in outbred animals using a continuous access, 2-bottle choice paradigm, and to contrast this intake with ontogenetic patterns of food and water intake. By comparing intake in adulthood of animals initially given access to ethanol in adolescence with those whose initial experiences with ethanol began in adulthood, these studies were also designed to determine whether extended home cage access to ethanol during adolescence elevates subsequent rates of voluntary ethanol drinking in adulthood.
MATERIALS AND METHODS
Subjects
A total of 46 male Sprague–Dawley rats (Taconic Farms) bred in our colony were used in this experiment. On P1, the day after birth, litters were culled to 8 to 10 pups, with 6 animals of one sex and 4 of the other kept whenever possible (and female offspring used in other projects in our laboratory). Offspring were weaned on P21, pair-housed with a same-sex littermate in a temperature-controlled vivarium on a 14:10 light/dark cycle (lights on at 7:00 am), and given ad libitum access to food (Purina Rat Chow, Lowell, MA) and water. At all times, animals were treated in accordance with guidelines for animal care established by the National Institutes of Health (Institute of Laboratory Animal Resources, Commission on Life Science, 1996).
Procedure
A 2 age (adolescent or adult)×3 solution (EtOH, sweetened EtOH, or saccharin) factorial design was used, with 8 animals placed into each experimental group and no more than 1 animal per litter placed into any experimental condition. Starting in early adolescence (P27–28) or early adulthood (P70–71), rats were singly housed in standard clear plastic breeder tubs (24×45.4×20 cm) containing pine shavings. They were then given a 2-bottle free-access choice between water and 1 of 3 experimental solutions in their home cage. Experimental solutions included an ethanol solution (10% v/v), a sweetened ethanol solution (10% +0.1% w/v saccharin), and a saccharin solution (0.1% w/v). Tap water was used to prepare all solutions and the positions of the 2 bottles were rotated daily to avoid a side preference. Both water and the experimental solutions were presented in 100 mL graduated glass bottles (Ancare, Bellmore, NY), with BP sipper tubes. Animals were given access to the solutions for approximately 23 hours a day, from 3:00 pm to 2:00 pm. At that time, both bottles were removed, solution and food intakes were recorded, and animals were weighed before returning fresh solutions to the cage. Intake was recorded for 63 days in animals initiated in adolescence (P28–90) and for 20 days in animals whose exposure began in adulthood (P71–90).
Statistics
Data were analyzed across days using within-subjects analyses of variance (ANOVAs), followed by post hoc contrasts with Fisher’s LSD tests to determine the locus of significant main effects and interactions. Levene’s tests were used to examine homogeneity of variance within the data set, with data violating the assumption transformed as necessary before analysis by ANOVA (for ease in interpreting figures, however, nontransformed data are shown in all graphical representations of the data). For assessment of age differences in intake, data from P28 to 41 in adolescents and a comparable period in adulthood (P71–84) were blocked into 2-day blocks before analysis via ANOVA. For assessment of intake in adolescent-initiated animals throughout the adolescent-to-adult transition period, data over the 63-day intake period (i.e., P28–90) were transformed into 21, 3-day blocks. For comparisons between adolescent-initiated and adult-initiated animals, data gathered from P71 to 90 in each group were analyzed in 2-day blocks.
RESULTS
Age Differences in Intake
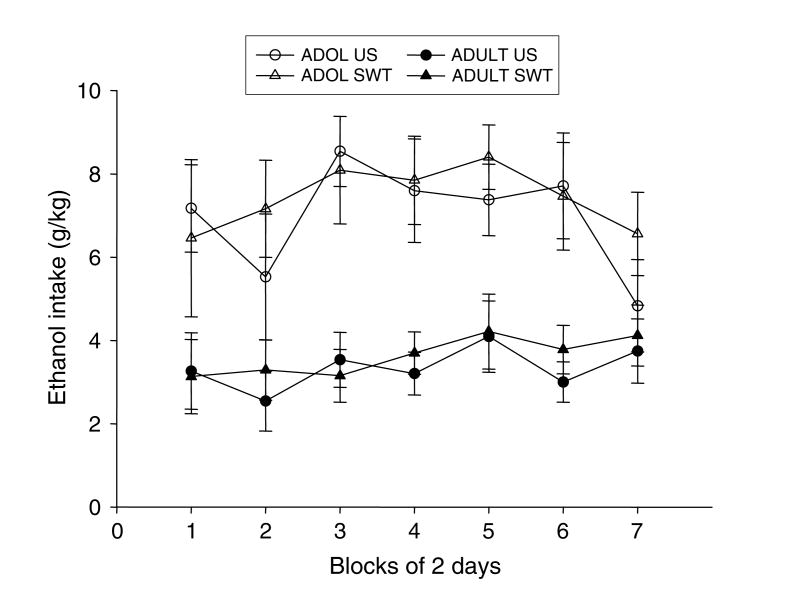
Ethanol Intake (g/kg) (Fig. 1)
Fig. 1.
Mean intake (g/kg) of unsweetened (US) and sweetened (SWT) ethanol by both adolescent (ADOL) and adult (ADULT) rats across the seven 2-d blocks of measurement. Scale bars represent standard errors, as in all other graphs in this experiment.
The gram per kilogram intake data violated the assumption of homogeneity of variance, and were therefore subjected to a log(10) (n+1) transformation before analysis via a 2 age × 2 solution (sweetened vs unsweetened EtOH) × 7 block ANOVA. Adolescent animals consumed significantly more ethanol (g/kg) than adult animals [main effect of age: F(1, 28) = 18.42, p≤0.001], with adolescent intake levels reaching 8 g/kg/d. Intake levels increased to a plateau and then declined slightly across the measurement period [main effect of block: (9, 252) = 2.13, p≤0.05]; although this effect was seemingly driven by the adolescent animals, the block×age interaction did not reach significance (p = 0.19). As can be seen in Fig. 1, the sweetener did not influence ethanol intake, with no significant main effect or interactions involving sweetener in this analysis.
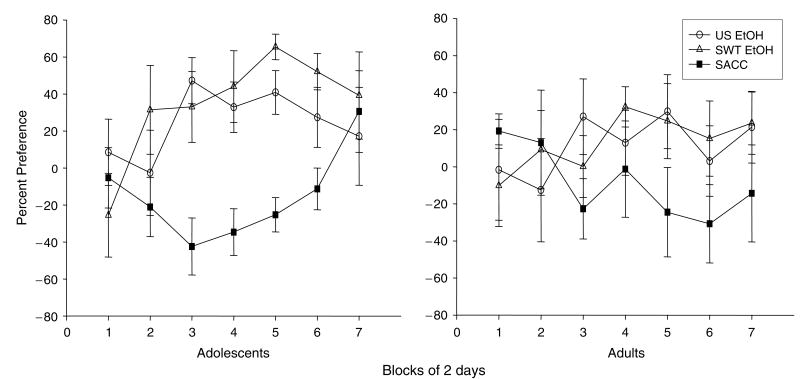
Percent Preference (Fig. 2)
Fig. 2.
Percent preference for the 3 experimental solutions: unsweetened ethanol (US EtOH), sweetened ethanol (SWT EtOH), or saccharin (SACC) relative to water in adolescent and adult rats across the seven 2-d blocks of measurement.
Preference scores were calculated via the formula: (mL experimental solution intake − mL water intake)/(mL experimental solution intake+mL water intake)×100. Using this means of calculating preference scores, values greater than zero reflect a preference for the experimental solution, with scores below zero reflecting a water preference. Overall, both ethanol solutions were preferred over water, whereas saccharin was not, although this main effect of solution [F(2, 40) = 4.93, p≤0.01] was tempered by a significant block×solution interaction [F(18, 360) = 2.75, p≤0.01]. Fisher’s post hoc analysis on data collapsed across age to explore this interaction revealed that it took several blocks for preference patterns to emerge, with preference for the sweetened ethanol solution during the first block being significantly lower than all subsequent blocks, whereas preference for the unsweetened ethanol solution during the first and second blocks was significantly lower than the third and fifth blocks. No effects of age were found.
Body Weight Gain (Not Shown)
Comparisons of weight gain across age were made by converting body weight into a percent change from the previous day using the formula: [(day(n+1) weight − dayn weight)/dayn weight]×100. Showing a typical pattern of hyperphagia, adolescent animals gained a significantly greater percentage of their body weight daily (5.26 ± 0.07) than adult animals (0.92 ± 1.12), as reflected by a main effect of age [F(1, 40) = 678.08, p ≤0.001]. A main effect of block was also evident [F(9, 360) = 2.61, p ≤0.05], with body weight gain diminishing across time; although this effect appeared to be more pronounced in adolescents, the block×age interaction did not reach significance (p = 0.14). No effect of experimental solution on body weight gain was observed.
Intake Throughout the Adolescent-to-Adult Transition
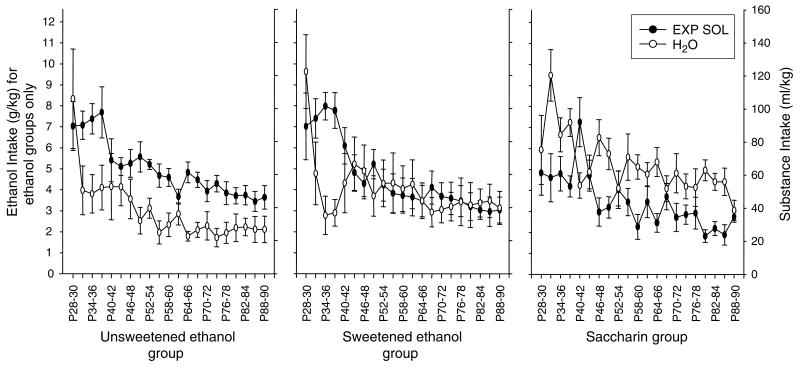
Ethanol Intake (g/kg) in Adolescent Animals (Fig. 3, left two panels)
Fig. 3.
Intake of experimental solution (EXP SOL) versus water (H2O) in animals examined throughout adolescence and into adulthood. The left y-axis provides values for gram per kilogram intake and is relevant to the ethanol intake data only, whereas the right y-axis reflects milliliter per kilogram intake for all fluids and groups.
Over the 63-day test period, adolescent ethanol intake decreased significantly [main effect of block: F(1, 20) = 13.34, p≤0.01], with adult-like levels of intake reached by approximately P70 (i.e., block 15). Ethanol intake during the early days of measurement (i.e., the first four 3-day blocks: P28–39) was significantly greater than intake during all later measurements. Following a notable decline after this plateau, intake declined more gradually across days, with intake during blocks 5–9 (i.e., P40–54) significantly elevated compared with intake during the last 4 blocks (i.e., P79–90). Thus, developmentally, ethanol intake is the greatest early in adolescence, declining in a step-like fashion later in adolescence and then more gradually into adulthood.
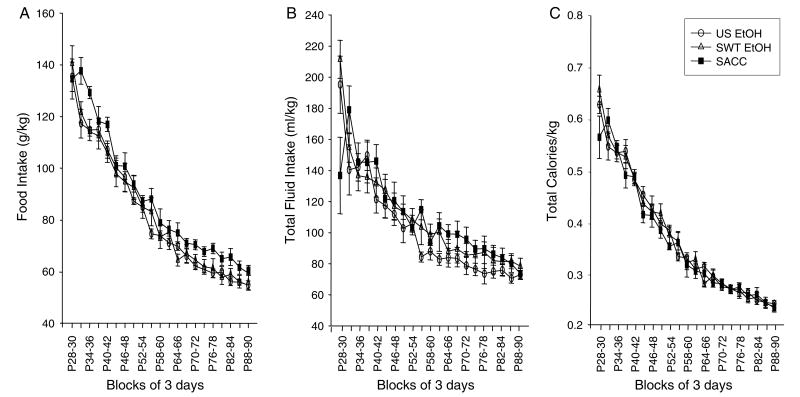
Food and Total Calorie Intake in Adolescent Animals (Fig. 4A and 4C)
Fig. 4.
Food intake (A), total fluid (B), and total caloric intake (C) per kilogram of body weight for each experimental group: unsweetened ethanol (US EtOH), sweetened ethanol (SWT EtOH), and saccharin (SACC), in animals examined throughout adolescence and into adulthood.
Food intake per kilogram of body weight was determined via the formula: [(dayn food weight (g) – day(n+1) food weight (g))/dayn body weight (kg)]. Food intake of animals tested beginning early in adolescence showed a gradual ontogenetic decline [main effect of block: F(20, 420) = 185.79, p≤0.001] (Fig. 4A). This gradual decline varies considerably from the more step-like pattern seen with ethanol intake. A main effect of solution [F(2, 21) = 3.63, p≤0.05] was also observed, with animals given access to the saccharin solution consuming more food than animals receiving either ethanol solution. When intake data were analyzed for total calories consumed per kilogram body weight via the formula: [((dayn ethanol solution intake (g)×7 calories/g of ethanol)+(dayn food intake (g)×4.01 calories/g of food))/(dayn body weight (kg))] (see Fig. 4C), no significant differences between solutions emerged; thus, the lower food intake evident among animals receiving either ethanol solution was likely due to the caloric content of the ethanol. Again, animals showed a gradual decline in total calorie intake across the 63-day measurement period [main effect of block: F(1, 20) = 212.73, p≤0.001].
Total Fluid Intake (mL/kg) in Adolescent Animals (Fig. 3B)
Total fluid intake was calculated via the formula: [(dayn water intake (mL)+dayn experimental solution in-take (mL)/(dayn body weight (kg)]. In the ANOVA of total fluid intake across the adolescent-to-adult transition, a significant block×solution interaction emerged [F(40, 420) = 2.21, p ≤ 0.001]. Fisher’s post hoc analyses revealed that the interaction appeared to be mainly driven by the first block of intake wherein animals in the ethanol groups tended to drink more overall fluid than animals in the saccharin group, an effect that reached significance in the sweetened ethanol group. No other differences were seen across solution condition on any day. Although the total fluid data were somewhat more variable than the analyses of food and calorie intake, the total intake of fluids gradually declined across the adolescent-to-adult transition.
Ontogenetic Pattern of Water Versus Experimental Fluid Intake (ml/kg) in Adolescent Animals (Fig. 3)
To directly compare ontogenetic patterns of water and intake of experimental solutions during the adolescent-to-adult transition, milliliter per kilogram intake was compared using a 3 group (saccharin, unsweetened EtOH, or sweetened EtOH)×2 substance (experimental solution vs water)×21 block ANOVA. A significant group×substance×block interaction was revealed [F(40, 840) = 2.34, p≤0.001]. Fisher’s post hoc analyses showed that animals given access to unsweetened ethanol consumed significantly more of the ethanol solution than water during blocks 2 to 4 (i.e., P31–39); a similar effect was seen in the sweetened ethanol group, with ethanol intake significantly enhanced over water intake during blocks 3 and 4 (P34–39). For the remainder of the measurement period, ethanol consumption was significantly higher relative to water in the unsweetened ethanol group for approximately half of the measurement days while in the sweetened ethanol group, intake was similar to that of water. In contrast, animals in the saccharin group consumed significantly more water than saccharin solution for approximately half of the measurement days without a noticeable plateau during blocks 3 and 4 as seen in the ethanol groups.
Impact of Age of Initial Exposure on Ethanol Intake in Adulthood
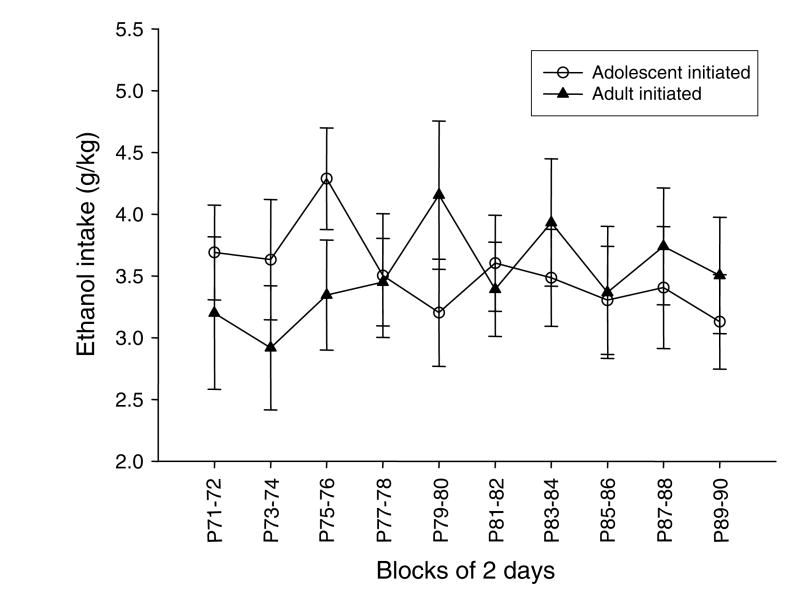
Ethanol Intake (g/kg) (Fig. 5)
Fig. 5.
Ethanol intake (g/kg) of animals initiated during adolescence and during adulthood at the same chronological age (P71–90). As no differences in intake between ethanol groups (i.e., unsweetened and sweetened) were observed during analysis, the ethanol data are collapsed across sweetener for graphical purposes.
Ethanol intake of animals initiated during adolescence was compared across the same chronological age as animals initiated during adulthood using a 2 (age)×2 (solution)×10 (block) design. A significant block×age interaction emerged in this ANOVA [F(9, 252) = 2.03, p≤0.05], although Fisher’s post hoc tests revealed no meaningful significant differences between the age of initiation groups within any of the blocks nor across days within either initiation age group. Animals who were given access to ethanol beginning in adolescence did not differ in their ethanol intake from animals whose ethanol access did not begin until adulthood.
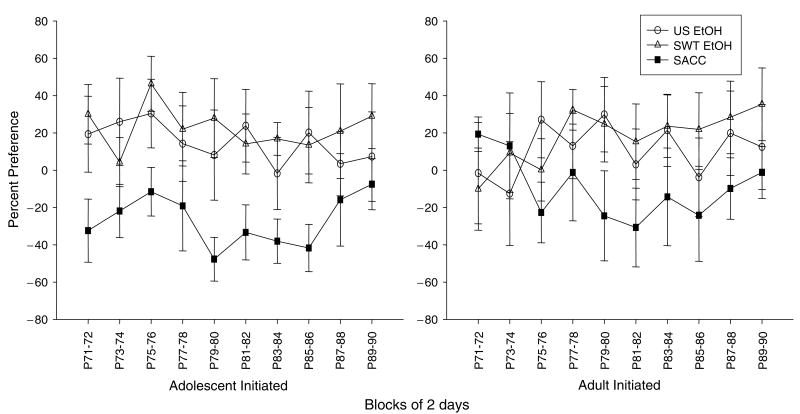
Percent Preference (Fig. 6)
Fig. 6.
Percent preference for experimental solutions: unsweetened ethanol (US EtOH), sweetened ethanol (SWT EtOH), or saccharin (SACC) compared with water in animals initiated during adolescence and adulthood across the ten 2-d blocks of measurement.
Regardless of age of initiation, both ethanol solutions (unsweetened ethanol and sweetened ethanol) generally yielded positive preference scores whereas the saccharin solution did not [main effect of solution: F(2, 40) = 3.88, p≤0.05]. There were no effects of age of initiation; thus, animals who began testing during adolescence showed similar preferences as animals given access beginning in adulthood.
DISCUSSION
Adolescent animals consumed significantly more ethanol (g/kg) than their adult counterparts during the first 2 weeks of comparison, with voluntary intakes of sweetened or unsweetened ethanol among adolescents peaking at an average of 7.5 g/kg/d, almost twice that of adults. The levels of ethanol intake observed in adolescents in this study were similar to those seen in experiments examining adolescent and adult animals from genetically selected rodent lines such as HAD and P rats in 2-bottle choice continuous-access paradigms (McKinzie et al., 1996). These results replicate previous findings in our laboratory of elevated ethanol intake (2–3 times higher relative to body weight than adults) during adolescence under a variety of experimental conditions in Sprague–Dawley rats (Brunell and Spear, 2005; Doremus et al., 2005).
Using a strategy for calculating preference scores whereby positive scores indicate a preference for the experimental solution and negative scores reflect a water preference (for the rationale, see Doremus et al., 2005), animals at both ages preferred the ethanol solutions over water whereas water was generally preferred over the saccharin alone solution. This effect, however, was tempered by block, and tended to be driven more strongly by the adolescents (see Fig. 2). These findings contrast with observations that under some experimental conditions, preference for unsweetened ethanol over water does not peak until adulthood in Sprague–Dawley rats (Goodrick, 1967; Parisella and Pritham, 1964), or that adolescent Wistar rats had preference scores in the aversive range whereas adults preferred an unsweetened ethanol solution during the first 4 days of self-administration (Siegmund et al., 2005). Previous work in our laboratory using Sprague–Dawley rats, however, has also found increased ethanol preference over water and other experimental solutions during adolescence under certain circumstances, especially when solutions were presented through BP sipper tubes as in the present study (Doremus et al., 2005).
Transient increases in total fluid and food consumption, referred to as hyperdipsia and hyperphagia, respectively, are characteristic of the adolescent growth spurt (Nance, 1983), and could contribute to increases in ethanol consumption seen during this ontogenetic period. However, based on the results of the present study, adolescent-typical hyperdipsia and hyperphagia cannot fully explain the elevated ethanol intake of adolescents, given that the ontogenetic pattern of ethanol intake during the adolescent-to-adult transition varies from that seen in terms of the intake of food and water, as well as total caloric consumption. Consumption of the ethanol solutions showed a clearly elevated plateau during the first 2 weeks of measurement (i.e., blocks 1–4: P28–39) in early adolescence (see Fig. 3), before declining sharply at approximately P40 (i.e., block 5) to levels that were only modestly elevated compared with the adult-typical consumption pattern that was reached by approximately P70 (i.e., block 15). This pattern was not evident with saccharin consumption, with such intake showing only a gradual (albeit somewhat variable) decline with age. Likewise, food (see Fig. 4A), total fluid (see Fig. 4B), and total caloric (see Fig. 4C) intake generally declined gradually into adulthood, although the data are more variable, and ontogenetic patterns less clear with total fluid intake.
Age-related neurobehavioral differences in sensitivity to ethanol within adolescence could have contributed to the elevated levels of ethanol intake observed during early-to-mid adolescence. For instance, developmental reorganization of the dopamine (DA) system during adolescence has been suggested to alter the balance of DA activity from prefrontal predominance in early adolescence to a shift toward greater DA activity in striatal and mesolimbic regions late in adolescence (Andersen, 2003; Spear, 2000). Given the importance of the nucleus accumbens for modulating the salience of rewarding stimuli, including alcohol and other addictive drugs (Koob, 1992), relatively lower basal DA activity in the nucleus accumbens early in adolescence could potentially contribute to the elevated ethanol intake observed in the present experiment during the early-to-mid adolescent period. Work in our laboratory in Sprague–Dawley rats has also found age-related differences in ethanol-induced social facilitation, with this increased sensitivity to the stimulating effects of ethanol on social behavior being more pronounced in early than mid adolescence (Varlinskaya and Spear, 2006a, 2006b). It has been suggested that this difference may stem in part from differential sensitivity to ethanol-induced changes in endogenous opioid systems during adolescence (Varlinskaya and Spear, 2006a, 2006b). However, it remains to be determined whether differences in the sensitivity of endogenous opioid systems or a transient developmental attenuation in accumbal DA activity contribute to the elevations in ethanol consumption and preference seen during early-to-mid adolescence relative to later in adolescence or adulthood.
It is possible that an adolescent insensitivity to aversive and dysphoric effects of ethanol (e.g., motor impairing effects, sedative/hypnotic effects) (Silveri and Spear, 1998, 2001) may act as a permissive factor, allowing adolescents to consume more ethanol before experiencing feedback cues that would moderate consumption (see Spear and Varlinskaya, 2005 for a review).
Age-related pharmacokinetic factors might also contribute to the increased intake of adolescents relative to adults. There is some evidence for slightly faster ethanol elimination rates in adolescent Sprague–Dawley rats than adults when ethanol was injected intraperitoneally (Silveri and Spear, 2000). In contrast, when an ethanol in milk solution was administered intragastrically to adolescent Sprague–Dawley rats, they showed slightly slower ethanol clearance than rats in early adulthood (Kelly et al., 1987). Ethanol metabolism has not been systematically examined following self-administered ethanol within the adolescent period, however, and hence it remains to be determined whether ontogenetic differences in ethanol metabolism contribute to the plateau pattern and subsequent decline in ethanol consumption that occur during the early-to-late adolescent transition.
Another possible contributor to the elevated intake observed in adolescent animals is an increased response to novelty. Several studies have shown that adolescent Sprague–Dawley rats show greater novelty preference, higher novelty-induced locomotor activity, and increased exploration of novel stimuli than adult animals (Douglas et al., 2003; Stansfield and Kirstein, 2005). Under the present paradigm, animals were exposed to 2 novel stimuli upon initiation of the experiment: presentation of all fluids solution through BP sipper tubes and exposure to the experimental solution itself. It is possible that increased exploration of the BP sipper tube paired with the novelty of the ethanol solution by adolescents could have led to increased intake of the solution. However, adolescent-specific increases in response to novelty do not fully explain the plateau pattern observed in adolescent ethanol intake, given that animals with access to the equally novel saccharin solution did not show the same pattern.
When ethanol intake was assessed in adulthood, animals given ethanol access beginning in adolescence drank similar amounts as animals not given ethanol access until adulthood. Preferences for ethanol were also similar in animals initiating ethanol consumption in adolescence when compared with animals beginning consumption in adulthood. It is possible that differences between adolescent-initiated and adult-initiated groups might have emerged in the current study if adult-initiated animals had been exposed to ethanol as long as the adolescent-initiated group, although this possibility seems unlikely, given the relatively stable levels of intake observed in both groups when examined in early adulthood. Moreover, these findings of equivalent intake regardless of initiation age are consistent with work showing that early forced exposure to 10% ethanol for 3 to 10 days during adolescence in rats did not enhance ethanol’s reinforcing properties (as indexed by operant self-administration) in adulthood (Tolliver and Samson, 1991). Forced exposure to ethanol vapors in adolescent Sprague–Dawley rats also did not enhance ethanol drinking in adult animals (Slawecki and Betancourt, 2002). However, the results of the present study contrast with studies conducted in C57BL/6J mice where postweaning 2-bottle choice exposure slightly increased ethanol consumption and preference in adulthood (Blizard et al., 2004; Ho et al., 1989), as well as with reports that rats bred for high levels of alcohol consumption (HAD rats) given chronic access to ethanol beginning during adolescence exhibited greater consumption levels than HAD rats not given this access as adolescents (Bell et al., 2004). Specific test parameters (i.e., exact age of initiation, tube type, housing condition) could explain the variations in findings across these studies.
The development of alcohol use disorders in adulthood is strongly influenced by genetic factors, with estimates that a combination of multiple genes accounts for approximately 50 to 60% of the vulnerability for development of alcohol use disorders (Enoch and Goldman, 2001; McGue, 1999). However, these genetic influences interact with and are modulated by environmental factors (Rose, 1998). Traditional animal models of genetically influenced drinking have utilized rodents selectively bred for the behavioral phenotype of high alcohol drinking and preference such as P and HAD rats. Genetic contributors to high ethanol drinking are evident at an early age, with adolescent P rats consuming more ethanol than their nonpreferring counterparts (McKinzie et al., 1998). Similarly, among the factors contributing to problem drinking in human adolescents is a family history of alcoholism. For instance, adolescents with a family history of alcoholism were more likely to exhibit elevated alcohol drinking (whether adolescent-limited or escalating beyond adolescence) than to be placed in a “no or low problem” group (Warner et al., 2007). It is possible that the use of outbred rats as in the present study may not have modeled problematic patterns of adolescent alcohol drinking sufficiently to have resulted in the maintenance or escalation of high levels of alcohol intake into adulthood.
Although ethanol consumption levels reached early in adolescence (7.5 g/kg/d) approached those seen in P rats (Files et al., 1992), blood ethanol concentrations were not assessed in this study due to difficulties in pin-pointing the specific timing of drinking bouts that are interspersed throughout the dark cycle (and episodically continued through the light cycle as well) in both adolescent and adult Sprague–Dawley rats (see Brunell and Spear, 2005). Given the episodic nature of home cage consumption (e.g., Brunell and Spear, 2005), it is possible that ethanol burdens at any point in time were not sufficiently high to exert a lasting influence on subsequent intake. That is, despite the overall high daily ethanol intakes of the adolescents, their blood ethanol concentrations may not have reached pharmacologically significant levels or levels sufficient to induce tolerance, and hence, may not have affected intake in adulthood. Given that episodic intake (e.g., “binge-drinking”) is particularly prevalent among adolescents (Johnston et al., 2003), it may be important to model the impact of more “binge”-like ethanol exposures during adolescence on later ethanol consumption and preference in adulthood in future work.
It is common for researchers to use nonnutritive sweeteners, such as saccharin, to maximize ethanol consumption levels in animal models of voluntary intake (Brunell and Spear, 2005; Doremus et al., 2005; Samson and Falk, 1974). It has also been shown that intake of saccharin-alone solutions is highly correlated with voluntary consumption of unsweetened ethanol solutions in adult rats and mice under a variety of circumstances in both alcohol-preferring and nonpreferring strains (Kampov-Polevoy et al., 1990; Sinclair et al., 1992). According to self-reports, human adolescents prefer low-ethanol, high-carbohydrate containing drinks such as beer, wine coolers, and sweetened mixed drinks (Wechsler et al., 2000; Substance Abuse and Mental Health Services Administration, 2001). Thus, it was expected that the addition of saccharin to the ethanol solution would enhance intake. Surprisingly, in the present experiment there was a lack of effect of sweetener on ethanol consumption or ethanol preference at either age. Similar results were found by Roberts et al. (1999) in a study examining male Wistar rats trained to lever press for unsweetened, saccharin-sweetened, or sucrose-sweetened ethanol solutions. Animals consumed more of an ethanol solution if it was sweetened (and calorically supplemented) with sucrose, but similar amounts when the solution was unsweetened or saccharin-sweetened. Saccharin, while mainly possessing sweet taste quality, has also been shown by in vitro data to have an intrinsically bitter aftertaste, presumably due to the activation of not only sweet taste receptors but also receptors sensitive to bitter components within human tongue taste papillae (Kuhn et al., 2004). There is some evidence that saccharin may have an aversive taste component in rats as well (Dess, 1993). Indeed, in the present study, animals preferred water over the saccharin solution in the saccharin-only group. In addition, adolescents given access to saccharin-sweetened ethanol did not show greater intake of the sweetened solution over water for as many blocks as did animals given access to unsweetened ethanol. It is possible that the slight attenuation in ethanol intake seen in the group receiving saccharin-sweetened ethanol may be in part a function of the aversive effects of the saccharin countering the positive pharmacological effects of ethanol.
In contrast to epidemiological studies showing age of onset of alcohol use to be predictive of increased risk for development of alcohol use disorders (Dewit et al., 2000; Grant and Dawson, 1997), voluntary ethanol intake throughout adolescence and into adulthood was not found to elevate intake in adulthood in the present study. There is some evidence in human studies that relatively high levels of alcohol intake during adolescence are often “adolescent-limited” and become more modest in adulthood (Bates and Labouvie, 1997). Average longitudinal patterns taken from the Rutgers Health and Human Development Project show that alcohol use and intensity increase steadily until about 18 to 21 years of age, followed by a leveling off during the early twenties and a decline during the late twenties (White et al., 1998). While age of initial alcohol exposure and use may be positively correlated with later alcohol abuse, such correlations do not prove causality, and it is possible that type of drinking behavior (e.g., binge drinking) and other behavioral risk factors may play a critical role in the continuation of alcohol use disorders into adulthood (Hill et al., 2000). We are currently exploring other models of ethanol consumption during adolescence, with a particular focus on modeling binge-like consumption patterns.
Acknowledgments
This research was supported by NIAAA Grant R37 AA12525.
References
- Adriani W, Macri S, Pacifici R, Laviola G. Peculiar vulnerability to nicotine oral self-administration in mice during early adolescence. Neuropsychopharmacology. 2002;27:212–224. doi: 10.1016/S0893-133X(02)00295-6. [DOI] [PubMed] [Google Scholar]
- Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Arnett J. Reckless behavior in adolescence: a developmental perspective. Dev Rev. 1992;12:339–373. [Google Scholar]
- Bates ME, Labouvie EW. Adolescent risk factors and the prediction of persistent alcohol and drug use into adulthood. Alcohol Clin Exp Res. 1997;21:944–950. [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Hsu CC, Lumeng L, Li TK, Murphy JM, McBride WJ. Effects of concurrent access to a single concentration or multiple concentrations of ethanol on ethanol intake by periadolescent high-alcohol-drinking rats. Alcohol. 2004;33:107–185. doi: 10.1016/j.alcohol.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Sable HJK, Schultz JA, Hsu CC, Lumeng L, Murphy JM, McBride WJ. Daily patterns of ethanol drinking in periadolescent and adult alcohol preferring (P) rats. Pharmacol Biochem Behav. 2006;83:35–46. doi: 10.1016/j.pbb.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Belluzzi JD, Lee AG, Oliff HS, Leslie FM. Age-dependent effects of nicotine on locomotor activity and conditioned place preference in rats. Psychopharmacology. 2004;174:389–395. doi: 10.1007/s00213-003-1758-6. [DOI] [PubMed] [Google Scholar]
- Blizard DA, Vanderbergh DJ, Jefferson AL, Chatlos CD, Vogler GP, McClearn GE. Effects of periadolescent ethanol exposure on alcohol preference in two BALB substrains. Alcohol. 2004;34:177–185. doi: 10.1016/j.alcohol.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Brunell SC, Spear LP. Effect of stress on the voluntary intake of a sweetened ethanol solution in pair-housed adolescent and adult rats. Alcohol Clin Exp Res. 2005;29:1641–1653. doi: 10.1097/01.alc.0000179382.64752.13. [DOI] [PubMed] [Google Scholar]
- Dess NK. Saccharin’s aversive taste in rats: evidence and implications. Neurosci Biobehav Rev. 1993;17:359–372. doi: 10.1016/s0149-7634(05)80113-7. [DOI] [PubMed] [Google Scholar]
- Dewit DJ, Adlaf EM, Offord DR, Ogborne AC. Age at first alcohol use: a risk factor for the development of alcohol disorders. Am J Psychiatry. 2000;157:745–750. doi: 10.1176/appi.ajp.157.5.745. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendron P, Spear LP. Factors influencing elevated ethanol consumption in adolescent versus adult rats. Alcohol Clin Exp Res. 2005;29:1796–1808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- Douglas LA, Varlinskaya EI, Spear LP. Novel-object place conditioning in adolescent and adult male and female rats: effects of social isolation. Physiol Behav. 2003;80:317–325. doi: 10.1016/j.physbeh.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Enoch MA, Goldman D. The genetics of alcoholism and alcohol abuse. Curr Psychiatr Rep. 2001;3:144–151. doi: 10.1007/s11920-001-0012-3. [DOI] [PubMed] [Google Scholar]
- Files FJ, Andrews CM, Samson HH, Lumeng L, Li TK. Alcohol self-administration in a nonrestricted access situation with alcohol-preferring (P) rats. Alcohol Clin Exp Res. 1992;16:751–756. doi: 10.1111/j.1530-0277.1992.tb00672.x. [DOI] [PubMed] [Google Scholar]
- Goodrick CL. Alcohol preference of the male Sprague–Dawley albino rat as a function of age. J Gerentol. 1967;22:369–371. doi: 10.1093/geronj/22.3.369. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from national longitudinal alcohol epidemiologic survey. J Subst Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Hill KG, White HR, Chung I, Hawkins JD, Catalano RF. Early adult outcomes of adolescent binge drinking: person- and variable-centered analyses of binge drinking trajectories. Alcohol Clin Exp Res. 2000;24:892–901. [PMC free article] [PubMed] [Google Scholar]
- Ho A, Chin AJ, Dole VP. Early experience and the consumption of alcohol by adult C57BL/6J mice. Alcohol. 1989;6:511–515. doi: 10.1016/0741-8329(89)90060-8. [DOI] [PubMed] [Google Scholar]
- Honey PL, Galef BG. Ethanol consumption by rat dams during gestation, lactation and weaning increases ethanol consumption by their adolescent young. Dev Psychobiol. 2003;42:252–260. doi: 10.1002/dev.10098. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG. Monitoring the Future National Results on Adolescent Drug Use: Overview of Key Findings, 2003. NIH Publication No. 03-5374. National Institute on Drug Abuse; Bethesda, MD: 2003. [Google Scholar]
- Johnston LD, O’Malley P, Bachman JG. The Monitoring the Future National Survey Results on Adolescent Drug Use: Overview of Key Findings, 2000. NIH Publication No 01-4923:1-60. National Institute on Drug Abuse; Bethesda, MD: 2001. [Google Scholar]
- Kampov-Polevoy AB, Kasheffskaya OP, Sinclair JD. Initial acceptance of ethanol: gustatory factors and patterns of alcohol drinking. Alcohol. 1990;7:83–85. doi: 10.1016/0741-8329(90)90065-k. [DOI] [PubMed] [Google Scholar]
- Kelly SJ, Bonthius DJ, West JR. Developmental changes in alcohol pharmacokinetics in rats. Alcohol Clin Exp Res. 1987;11:281–286. doi: 10.1111/j.1530-0277.1987.tb01308.x. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neural mechanisms of drug reinforcement. Ann NY Acad Sci. 1992;654:171–191. doi: 10.1111/j.1749-6632.1992.tb25966.x. [DOI] [PubMed] [Google Scholar]
- Kuhn C, Bufe B, Winnig M, Hofmann T, Frank O, Behrens M, Lewtschenko T, Slack JP, Ward CD, Meyerhof W. Bitter taste receptors for saccharin and acesulfame K. J Neurosci. 2004;24:10260–10265. doi: 10.1523/JNEUROSCI.1225-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster FE, Brown TD, Coker KL, Elliot JA, Wren SB. Sex differences in alcohol preference and drinking patterns emerge during the early postpubertal period. Alcohol Clin Exp Res. 1996;20:1043–1049. doi: 10.1111/j.1530-0277.1996.tb01945.x. [DOI] [PubMed] [Google Scholar]
- Maggs JL, Almeida DM, Galambos NL. Risky business: the paradoxical meaning of problem behavior for young adolescents. J Early Adolesc. 1995;15:344–362. [Google Scholar]
- McGue M. The behavioral genetics of alcoholism. Curr Direct Psychol Sci. 1999;8:109–115. [Google Scholar]
- McKinzie DL, Eha R, Murphy JM, McBride WJ, Lumeng L, Li TK. Effects of taste aversion training on the acquisition of alcohol drinking in adolescent P and HAD rat lines. Alcohol Clin Exp Res. 1996;20:682–687. doi: 10.1111/j.1530-0277.1996.tb01672.x. [DOI] [PubMed] [Google Scholar]
- McKinzie DL, Nowak KL, Murphy JM, Li TK, Lumeng L, McBride WJ. Development of alcohol drinking behavior in rat lines selectively bred for divergent alcohol preference. Alcohol Clin Exp Res. 1998;22:1584–1590. [PubMed] [Google Scholar]
- Nance DM. The development and neural determinants of the effects of estrogen on feeding behavior in the rat: a theoretical perspective. Neurosci Biobehav Rev. 1983;7:189–211. doi: 10.1016/0149-7634(83)90015-5. [DOI] [PubMed] [Google Scholar]
- Parisella RM, Pritham GH. Effects of age on alcohol preference by rats. Q J Stud Alcohol. 1964;25:248–252. [PubMed] [Google Scholar]
- Primus RJ, Kellogg CA. Pubertal-related changes influence the development of environmental-related social interaction in the male rat. Dev Psychobiol. 1989;22:633–643. doi: 10.1002/dev.420220608. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Heyser CJ, Koob GF. Operant self-administration of sweetened versus unsweetened ethanol: effects on blood alcohol levels. Alcohol Clin Exp Res. 1999;23:1151–1157. [PubMed] [Google Scholar]
- Rodd-Henricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, Li TK. Effects of ethanol exposure on subsequent acquisition and extinction of ethanol self-administration and expression of alcohol-seeking behavior in adult alcohol-preferring (P) rats: I. periadolescent exposure. Alcohol Clin Exp Res. 2002a;26:1632–1641. doi: 10.1097/01.ALC.0000036301.36192.BC. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, Li TK. Effects of ethanol exposure on subsequent acquisition and extinction of ethanol self-administration and expression of alcohol-seeking behavior in adult alcohol-preferring (P) rats: II. adult exposure. Alcohol Clin Exp Res. 2002b;26:1642–1652. doi: 10.1097/01.ALC.0000036302.73712.9D. [DOI] [PubMed] [Google Scholar]
- Rose RJ. A developmental behavior-genetic perspective on alcoholism risk. Alcohol Health Res World. 1998;22:131–143. [PMC free article] [PubMed] [Google Scholar]
- Samson HH, Falk JL. Schedule-induced ethanol polydipsia: enhancement by saccharin. Pharmacol Biochem Behav. 1974;2:835–838. doi: 10.1016/0091-3057(74)90118-x. [DOI] [PubMed] [Google Scholar]
- Siciliano D, Smith RF. Periadolescent alcohol alters adult behavioral characteristics in the rat. Physiol Behav. 2001;74:637–643. doi: 10.1016/s0031-9384(01)00623-0. [DOI] [PubMed] [Google Scholar]
- Siegmund S, Vengeliene V, Singer MV, Spanagel R. Influence of age at drinking onset on long-term ethanol self-administration with deprivation and stress phases. Alcohol Clin Exp Res. 2005;29:1139–1145. doi: 10.1097/01.alc.0000171928.40418.46. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcohol Clin Exp Res. 1998;22:670–676. doi: 10.1111/j.1530-0277.1998.tb04310.x. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Ontogeny of ethanol elimination and ethanol-induced hypothermia. Alcohol. 2000;20:45–53. doi: 10.1016/s0741-8329(99)00055-5. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Acute, rapid and chronic tolerance during ontogeny: Observations when equating ethanol perturbation across age. Alcohol Clin Exp Res. 2001;25:1301–1308. [PubMed] [Google Scholar]
- Sinclair JD, Kampov-Polevoy A, Stewart R, Li TK. Taste preferences in rat lines selected for low and high alcohol consumption. Alcohol. 1992;9:155–160. doi: 10.1016/0741-8329(92)90027-8. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Betancourt M. Effects of adolescent ethanol exposure on ethanol consumption in adult rats. Alcohol. 2002;26:23–31. doi: 10.1016/s0741-8329(01)00192-6. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP, Shalaby IA, Brick J. Chronic administration of haloperidol during development: behavioral and psychopharmacological effects. Psychopharmacology. 1980;70:47–58. doi: 10.1007/BF00432369. [DOI] [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI. Adolescence. Alcohol sensitivity, tolerance, and intake. Recent Dev Alcohol. 2005;17:143–159. [PubMed] [Google Scholar]
- Stansfield KH, Kirstein CL. Effects of novelty on behavior in the adolescent and adult rat. Dev Psychobiol. 2005;48:10–15. doi: 10.1002/dev.20127. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration . Summary of Findings From the 1999 National Household Survey on Drug Abuse. DHHS Publication No. (SMA) 00-3466. U.S. Department of Health and Human Services; Rockville, MD: 2001. [Google Scholar]
- Tolliver GA, Samson HH. The influence of early postweaning ethanol exposure on oral self-administration behavior in the rat. Pharmacol Biochem Behav. 1991;38:575–580. doi: 10.1016/0091-3057(91)90016-u. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Niesink RJM, Van Ree JM. The neurobiology of social play behavior in rats. Neurosci Biobehav Rev. 1997;21:309–326. doi: 10.1016/s0149-7634(96)00020-6. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Changes in sensitivity to ethanol-induced social facilitation and social inhibition from early to late adolescence. Ann NY Acad Sci. 2004;1021:455–461. doi: 10.1196/annals.1308.064. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Differences in social consequences of ethanol emerge during the course of adolescence in rats: social facilitation, social inhibition, and anxiolysis. Dev Psychobiol. 2006a;48:146–161. doi: 10.1002/dev.20124. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Ethanol-induced social facilitation in adolescent rats: role of endogenous activity at mu opioid receptors. Paper Presented at the Poster Session at the Annual Meeting of the Research Society on Alcoholism; Washington, DC. 2006b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner LA, White HR, Johnson V. Alcohol initiation experiences and family history of alcoholism as predictors of problem-drinking trajectories. J Stud Alcohol. 2007;68:56–65. doi: 10.15288/jsad.2007.68.56. [DOI] [PubMed] [Google Scholar]
- Wechsler H, Kuo M, Lee H, Dowdall GW. Environmental correlates of underage alcohol use and related problems of college students. Am J Prev Med. 2000;19:24–29. doi: 10.1016/s0749-3797(00)00163-x. [DOI] [PubMed] [Google Scholar]
- White HR, Bates ME, Labouvie E. In: Adult outcomes of adolescent drug use: a comparison of process-oriented and incremental analyses, in New Perspectives on Adolescent Risk Behavior. Jessor R, editor. Cambridge University Press; New York: 1998. pp. 150–181. [Google Scholar]