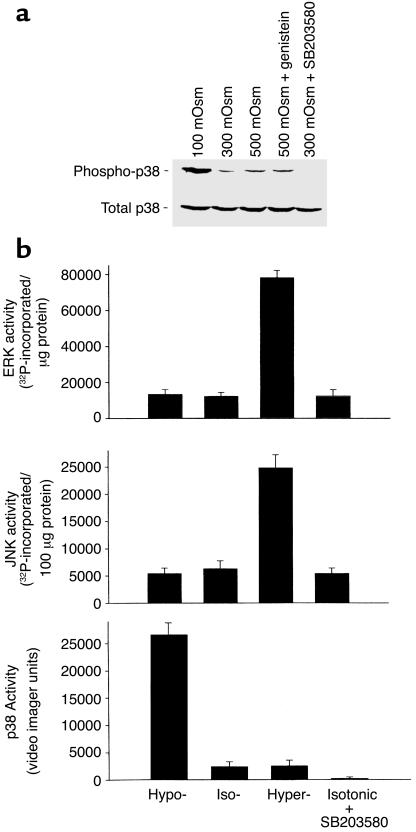
Figure 3.
Osmolarity-sensitive changes in MAP kinase activity. HTC cells were exposed to isotonic (∼300 mOsm), hypotonic (∼100 mOsm), or hypertonic (∼500 mOsm) buffer solutions for 5 minutes, then immediately homogenized in lysis buffer. (a) Cell lysates were subjected to SDS-PAGE and Western blot analysis with either an antibody specific for the activated (phosphorylated at Thr-180 and Tyr-182) form of p38 (phospho-p38) or a control antibody that does not distinguish between phospho- and dephosphorylated p38 MAP kinase (total p38). Constitutive phospho-p38 activity was observed under isotonic conditions; values increased with hypotonic exposure. Activity was inhibited by the p38 inhibitor SB203580 (1 μM), but not by the tyrosine kinase inhibitor genistein (10 μM). None of the various exposures resulted in changes in total p38. (b) Average MAP kinase activity in response to osmotic changes performed as described in Methods. Under isotonic conditions, there was constitutive activity of p38, JNK, and ERK kinases. Hypertonic exposure stimulated a large increase in both ERK (fivefold) and JNK (fourfold) activity, but had little effect on p38 MAP kinase activity. In contrast, hypotonic exposure resulted in a large increase in p38 MAP kinase activity (tenfold), but had little effect on ERK or JNK activity compared with isotonic conditions. The putative p38 MAP kinase inhibitor SB203580 did not affect ERK or JNK activity, but completely inhibited constitutive p38 MAP kinase activity.