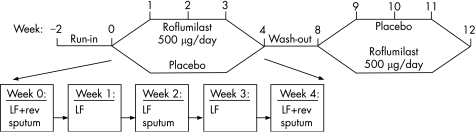
Figure 1 Following a 2‐week run‐in period, patients were randomised to receive roflumilast (500 μg once daily) or matching placebo for a period of 4 weeks. Four to 6 weeks after washout of the medication of the first treatment period, patients crossed over to the alternative treatment. During each treatment period, patients visited the department on a weekly basis. LF, lung function measurement by spirometry; rev, reversibility testing of forced expiratory volume in 1 s (FEV1) with 400 μg salbutamol.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.