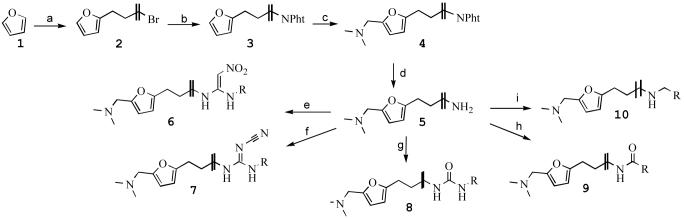
Scheme 1.
Reagents and conditions: (a) (i) BuLi, THF, 45°C. (ii) Br(CH2)4Br, THF, H2O, −30 °C → RT, 67% (b) KNPht, DMF, RT, 98% (c) HCHO, (CH3)2NH2Cl, EtOH, reflux, 16 h, 48%. (d) N2H4, NaOH, EtOH, reflux, 16h, 98%. (e) (i) 1,1-bis(methylthio)-2-nitroethylene, MeCN, Δ, 16h, 81%. (ii) MeNH2, EtOH, reflux, 16 h, 83% (f) (i) dimethyl cyanocarbonodithioimidate, EtOH, reflux, 16h, 76% (ii) MeNH2, EtOH, reflux, 16h, 88%. (g) RNCO, CH3CN, RT, 16h, 43%. (h) RCOCl, TEA, DCM, RT, 16h, 33%.(i) RCHO, MeOH, NaBH4, 5h, 95%.