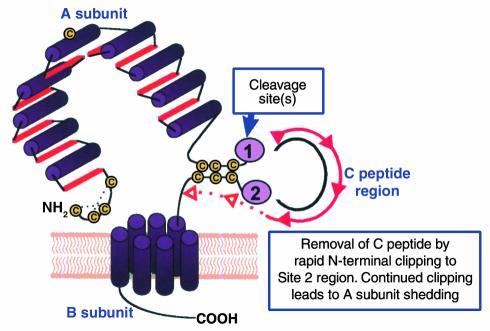
Figure 1.
Schematic representation of the TSHR with its large (397−amino acid residue without signal peptide) ectodomain, seven membrane-spanning segments, and short cytoplasmic tail. TSHR intramolecular cleavage into A and B subunits is associated with the loss of a C peptide region that corresponds approximately to a 50−amino acid “insertion” in the TSHR absent in the noncleaving LH and FSH receptors. The C peptide region is not removed intact. Following cleavage at upstream Site 1, the C peptide is rapidly degraded downstream to the Site 2 region. Evidence suggests that N-terminal degradation of the B subunit continues thereafter, leading to loss of the Cys residues tethering the A subunit and to shedding of the latter. The Cys-rich N-terminus of the A subunit is an important component of thyroid-stimulating autoantibodies and is likely to contain two disulfide bonds (hypothetically shown by dotted lines) contributing to a conformationally important portion of the molecule.