Abstract
During infection, human immunodeficiency virus type 1 integrase engages a number of molecules and mechanisms, both of viral and cellular origin. In one of such instances, integrase is degraded by the N-end rule proteasome pathway a process that targets the N-terminal residue of its substrates. Here we describe the properties of HIV-1 viruses in which the first amino acid residue of integrase has been substituted to render it resistant to the N-end rule pathway. As result of this exchange, we observe a set of class I and class II defects that result in a large decrease of viral replication efficiency. Specifically, reverse transcription and integration are the steps that appear to be affected. We propose that the severe deficiency of these mutants exert a strong selective pressure that leads to the near total conservation of the N-terminal residue of integrase in HIV-1, HIV-2 and SIV.
Keywords: HIV-1, Integrase, EGFP, N-end Rule
Introduction
Human immunodeficiency virus type 1 (HIV-1), as part of the retrovirus family, reverse transcribes its RNA genome and inserts the resulting cDNA into the chromosomal DNA of its host. This last process is mediated by the viral enzyme integrase (IN), in concert with cellular factors. In several studies (Engelman et al., 1995; Lu, Ghory, and Engelman, 2005; Lu et al., 2005a; Lu et al., 2005b; Wiskerchen and Muesing, 1995), the mutagenesis of conserved regions within IN has determined its importance in the various reactions that lead to integration (class I mutants) as well as in other steps of the viral life cycle, (class II mutants) (Engelman, 1999).
IN is the retroviral protease-dependent proteolytic product of the Gag-Pol polyprotein, and also one of the few known substrates for the ubiquitin/proteasome pathway known as N-end rule (Mulder and Muesing, 2000). The N-end-rule determines the half-life of its substrates by the identity of their first residue (Bachmair, Finley, and Varshavsky, 1986). In yeast, Rad6 and UBR1 are respectively the E2 conjugating enzyme and E3 ligase of this pathway (Bartel, Wunning, and Varshavsky, 1990; Dohmen et al., 1991; Madura, Dohmen, and Varshavsky, 1993). In mammalian cells, there are two Rad6 alleles, Rad6A and Rad6B (Koken et al., 1991), and at least 7 orthologs of UBR1 (Tasaki et al., 2005). Mammalian UBR1-2 and 4 have been shown to be involved in the degradation of ectopically expressed HIV-1 IN (Tasaki et al., 2005).
The first residue of HIV-1 IN is invariably a phenylalanine (Phe). Phe ranks as a primary instability determinant in the N-end rule hierarchy that classifies the N-terminal residues based on the (in)stability they provide to the rest of the protein (Varshavsky, 1997). In this report we determine the relevance of the first residue of HIV-1 IN in viral infection.
Results
Replication and infectivity
In order to assess the importance of IN N-terminal residue in HIV-1 infection, we mutated position 1 from Phe to Met, which enhances IN half-life in an isolated system (Mulder and Muesing, 2000). In addition, since changing the first residue of IN also alters the reverse transcriptase (RT) -IN cleavage site recognized by the viral protease, we made a second type of mutant by introducing a potentially compensatory substitution, from Leu to Met, at the C-terminal position of RT. This latter change results in a Met|Met combination, which is also found in HIV-1 at the junction between p2 and p7, and therefore more likely to be processed compared to the Leu|Met junction. As control for the Met|Met mutant, we constructed a virus carrying a Met|Phe combination.
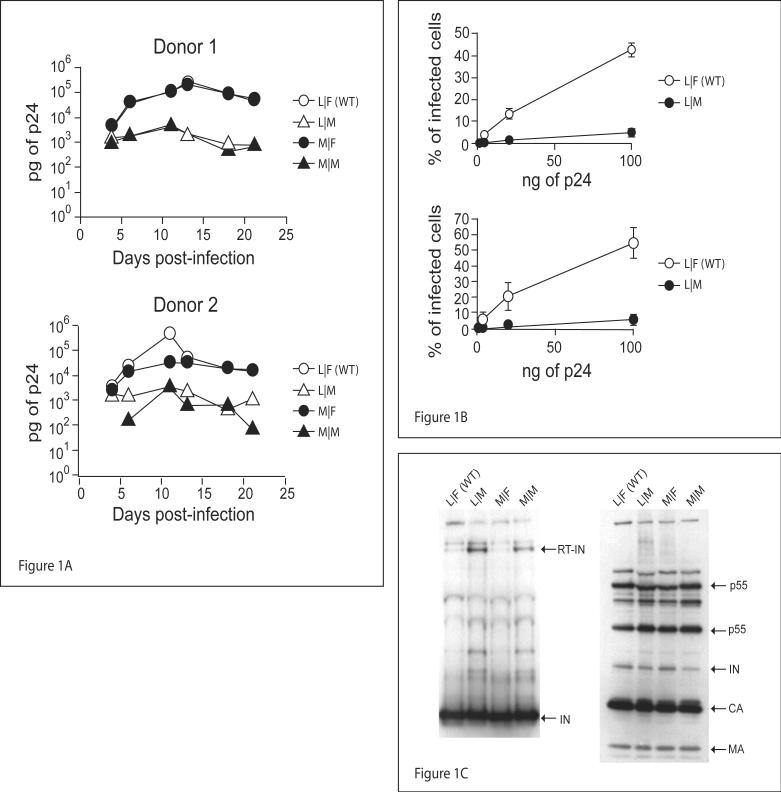
Viral replication was monitored by infecting PBMC from two different donors with equal amounts of either WT or mutant HIV-1 R7/3 viruses (figure 2A upper), and monitoring p24 values over a period of 21 days. As can be seen in figure 1A starting from day 6 post-infection, viruses with mutant IN have 10 to 100 fold lower replication rates than WT.
Figure 2. Reduced replication of integrase N-terminal mutant viruses is not due to a RT -IN cleavage defect.
A) Diagrams of the constructs used in this work. B) Concentrated R7/3 CMV EGFP ΔIN VSV-G pseudotyped viruses complemented with pLR2P-R-PC-IN and IN mutants were used to infect 40,000 TZM cells/well. Infectivity was measured by flow cytometry and indicated as percentage of EGFP positive cells 40-44 hours post-infection. C) Concentrated R7/3 CMV EGFP Δ IN VSV-G pseudotyped virions complemented with pLR2P-R-PC-IN and IN mutants were separated on a 10% polyacrylamide gel. Integrase was detected with monoclonal antibody 6G5.
Figure 1. Mutations of integrase N-terminal residue affect HIV-1 replication.
A) 2×106 PBMCs stimulated with PHA were infected with 50ng p24/well of R7/3 YU2 EGFP and IN mutants, p24 values were assessed over a period of 21 days post-infection. B) TZM cells plated at 40,000 cells/well in 24 well plates, were infected with 100ng/well and three 5 fold serial dilutions, of the WT and IN mutants of R7/3 CMV EGFP VSV-G pseudotyped virus. Infections were carried out overnight in the presence of 8μg/ml of polybrene. Infectivity was measured by flow cytometry and indicated as percentage of EGFP positive cells 40-44 hours post-infection. C) 490ng p24 of WT and IN mutant R7/3 CMV EGFP viruses were concentrated over a 20% sucrose cushion and half the amount loaded on a 10% polyacrylamide gel. Integrase was detected with monoclonal antibody 6G5 and HIV-1 proteins with patient anti-HIV serum.
To assess if the mutant viruses have a replication defect in the early phases of infection, TZM cells were infected with single round vesicular stomatitis virus glycoprotein (VSV-G) pseudotyped WT and mutant R7/3 CMV EGFP viruses (Figure 2A middle). Figure 1B shows that irrespective of the last residue of RT, viruses bearing a Met N-terminal residue in IN (Met-IN) are at least 8 fold (t-test p-value<0.05) less infectious than WT. To assess if mutant viruses contain quantities of IN similar to wild type, and can support a correct processing of the Gag-Pol polyprotein, equal amounts of concentrated virions were analyzed by western blot. As is shown in Figure 1C WT and mutant viruses contain comparable levels of IN, and Gag-Pol processing was equivalent in all the viral clones. Of note, though unlikely to play a major role, is that both viruses carrying mutant IN present a faint band at the level of the RT-IN polyprotein (Figure 1C first panel). In order to determine if this small amount of RT-IN could influence infectivity we decided to take advantage of a complementation system, described in (Fletcher et al., 1997), which allows to uncouple the proteolytic cleavage of RT-IN from the kinetic processing of HIV-1 polyproteins and from reverse transcription. In this system, the pLR2P-R-PC-IN plasmid (figure 2A lower) is used to ectopically express IN in the virus producer cell as a fusion with Vpr, which mediates proper incorporation and a short C-terminal stretch of RT encoding the RT-IN cleavage site. We complemented R7/3 CMV EGFP viruses lacking IN with the pLR2P-R-PC-IN plasmid encoding WT IN or its mutants. Figure 2B shows that, although infectivity is generally low, viruses with Met-IN are 4-5 fold (t-test p-values 0.0032 and 0.017 respectively) less infectious than the WT IN carrying controls. Also in this case, the quantities of IN present in the different virions are comparable (Figure 2C). Taken together these results show that the identity of the N-terminal residue of IN plays a significant role in HIV-1 infectivity, and that its influence exceeds that dictated by the optimal composition of the RT-IN proteolytic cleavage site.
Effects of IN N-terminal residue on reverse transcription
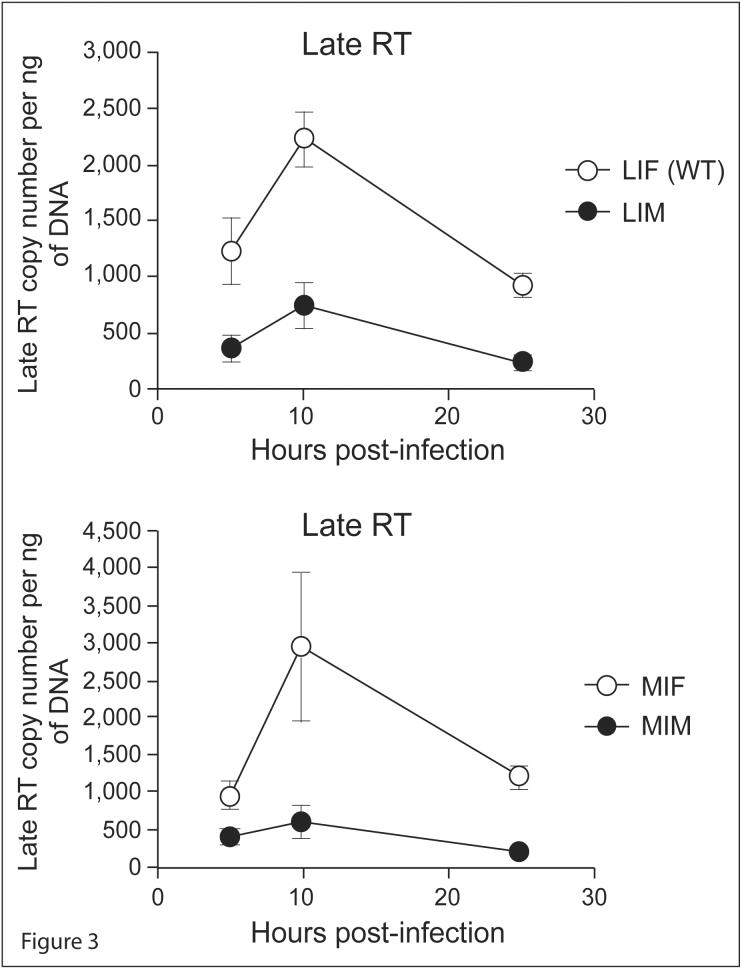
In order to determine which step between entry and integration is affected by the identity of the N-terminal residue of IN, we quantified the reverse transcription product of the different viruses at distinct time points. Viral cDNA was measured by fluorescent quantitative PCR (qPCR) using primers specific for late reverse transcription products. Figure 3 shows that Met-IN bearing viruses consistently exhibit a 2.5-5 fold (t-test p-value<0.01) reduced amount of late reverse transcription product than Phe-IN bearing viruses. These results suggest that the identity of the N-terminal residue of IN influences the synthesis or the accumulation of viral cDNA.
Figure 3. Integrase N-terminal mutant viruses have a defect in the accumulation of cDNA.
Cells were infected with 100ng WT and IN mutants of R7/3 CMV EGFP VSV-G pseudotyped virus for 5 hours and late reverse transcripts were assessed by qPCR at 5, 10, and 25 hours after infection.
Effect of IN N-terminal residue on integration
We next asked whether viruses with mutant INs are competent for integration or if a defect in this process could be an additional cause for their overall lower infectivity. We used an R7/3 CMV EGFP virus carrying an IN with a D116A active site substitution and complemented it with the pLR2P-R-PC-IN vector or its N-terminal mutants. Figure 4A shows that IN N-terminal mutants are only partially able to complement the catalytically inactive IN molecule of the backbone virus, with a 2-3 fold lower number of cells harboring the integrated provirus. Given that D116A IN mutant viruses are fully proficient for reverse transcription and that recombinant HIV-1 IN with an N-terminal Met is competent to perform concerted integration reactions in vitro, these results could arise from two causes: the most direct is that IN N-terminal mutants are less proficient in the integration reaction in vivo, but it is also possible that the mutants may have a dominant negative effect on reverse transcription, which would result in less substrate for integration. Since our previous results evidenced an influence of the N-terminal residue of IN on the accumulation of RT product we quantified the amount of viral cDNA synthesized after infection with WT and D116A viruses complemented with either N-terminal Met IN or WT IN. Figure 4B shows that levels of late RT product are comparable between viruses complemented either with WT IN or its N-terminal Met mutant (t-test p >0.05), and a 2 to 4 fold lower amount of late RT product in the complemented viruses compared to the non-complemented ones regardless of the identity of the N-terminal residue. These results indicate that an excess of IN has a detrimental effect on reverse transcription and that under these conditions the Met-IN does not add to the negative effect compared to WT IN likely because the amount of complementing WT IN is enough to overwhelm the system and accordingly overshadow any further effect due to IN stabilization. Taken together these data indicate that substitution of the N-terminal residue of HIV-1 IN results in the inability of the mutants to efficiently fulfill the integration step.
Figure 4. N-terminal mutant integrase only partially rescues integration-deficient HIV-1.
A) TZM cells were infected with pLR2P-R-PC-IN and IN mutants complemented R7/3 CMV EGFP D116A VSV-G pseudotyped viruses, split 1:10 and assessed by flow cytometry at 2, 6, and 10 days after infection. B) TZM cells were infected with pLR2P-R-PC-IN and IN mutants complemented R7/3 CMV EGFP and R7/3 CMV EGFP D116A VSV-G pseudotyped viruses for 5 hours and late reverse transcripts were assessed by qPCR at 10 hours after infection.
Discussion
Alongside RT, IN is the enzyme that distinguishes retroviruses from other viruses. IN belongs to a family of structurally related DNA transferases that includes phage transposases and mammalian VDJ recombinases RAG1 and RAG2. Recombinant HIV-1 IN has been shown to mediate a concerted in vitro integration reaction without any additional protein (Sinha, Pursley, and Grandgenett, 2002). Recently a number of host factors that interact with IN have been identified. Lens epithelium-derived growth factor (LEDGF) p75 has been identified as a primary IN binding protein (Cherepanov et al., 2003). The binding is lentiviral specific (Busschots et al., 2005; Llano et al., 2004) and LEDGF appears to tether IN to the host chromosomal DNA (Vanegas et al., 2005), thereby playing a role in site selection (Ciuffi et al., 2005) as well as facilitating integration (Vandekerckhove et al., 2006).
We have shown that HIV-1 IN interacts with DNA repair protein Rad18 (Mulder, Chakrabarti, and Muesing, 2002), and that cells lacking Rad18 are more permissive to viral infection (Lloyd et al., 2006).
HIV-1 IN is a substrate of the N-end rule proteasome pathway, in which the stability of a protein is determined by the identity of its N-terminal residue (Bachmair, Finley, and Varshavsky, 1986). HIV-1 IN is the product of the proteolytic cleavage of Gag-Pol, and has at its N-terminus a Phe, a highly destabilizing residue in the N-end rule. We therefore set out to test the relevance of this residue in the context of the HIV-1 early life cycle.
The results of our study indicate that the identity of IN N-terminal residue is of great importance as its substitution causes a near total inhibition of viral replication (Figure 1A). This outcome is the result of multiple defects mainly residing in the early phases of infection (Figure 1B) and involving both reverse transcription (Figure 3) and integration (Figure 4A). Evidences for a role of IN in reverse transcription have previously been described. Specifically it has been demonstrated that IN and RT directly interact in vitro (Tasara et al., 2001; Wu et al., 1999) and that a number of mutations in conserved regions of IN induce severe defects in viral cDNA synthesis (Ao et al., 2005; Lu, Ghory, and Engelman, 2005; Lu et al., 2005a; Wu et al., 1999). Our data imply that the identity of HIV-1 IN N-terminal residue influences reverse transcription, and also suggest that, because of the nature of the substitution analyzed such relevance might be related to the stability of integrase via the N-end rule proteasome pathway, as we reported earlier (Mulder and Muesing, 2000). Whether and how IN increased stability harms reverse transcription or the half-life of its product is currently under investigation. We speculate that higher concentrations of IN could directly inhibit RT as suggested by Tasara et al (Tasara et al., 2001), this is supported by the results shown in Figure 4B, where an excess of IN (from a complementation system in trans) decreased the amount of RT products. Alternatively, IN could be the target of host factors that can prevent it from efficiently carrying out its pro-reverse transcription activity.
The mechanism of integration inhibition by Met-IN is still unknown. Given that concerted integration can be efficiently achieved in vitro by recombinant Met-IN (Sinha, Pursley, and Grandgenett, 2002), we hypothesize that increased stability of integrase might be detrimental for the repair of the single stranded gap left after the insertion of the retroviral genome into the chromosomal DNA. Indeed, the prompt degradation of the integration complex might be necessary to allow the access of host derived DNA repair factors devoted to the closing of the gap. In this regard, it has been demonstrated that an excess of retroviral IN inhibits the access of repair polymerases to DNA gap repair reactions in vitro (Yoder and Bushman, 2000).
In conclusion we describe here that substitutions of the first residue of HIV-1 IN originate viruses with a complex phenotype, that can be classified both as class I mutants (Engelman, 1999), because of their inability to fully restore integration of integration-inactive viruses, as well as class II mutants because of their defect in the reverse transcription step. It is therefore not surprising, given the pleiotropic defects of the N-terminal IN mutant viruses, that the identity of IN first residue is almost absolutely conserved in HIV-1 and 2 and SIV (source: Los Alamos National Laboratory HIV Database).
Materials and methods
Plasmids and virus production
R7/3 YU2 EGFP (Wiskerchen and Muesing, 1995) (Figure 2A upper) was derived from the R7/3 HIV-1 clone (Feinberg et al., 1986) in which EGFP was cloned in the Nef position and the original envelope exchanged with an YU2 Env gene. R7/3 CMV EGFP (Figure 2A middle) was constructed by cloning the CMV EGFP expression cassette into the ENV position of the R7/3 clone. Vesicular stomatitis virus glycoprotein (VSV-G) was expressed from plasmid phCMV G (Yee, Friedmann, and Burns, 1994). IN complementation vector pLR2P-R-PC-IN (Figure 2A lower) was described earlier (Fletcher et al., 1997). IN point mutations were made by overlap PCR and using standard molecular biology techniques.
Viral stocks were made by transfecting HEK 293T with polyethylenimine (PEI) (Polysciences Inc.) (Durocher, Perret, and Kamen, 2002). Supernatants were harvested 40-44 hours later, 0.45μm filtered, aliquoted and frozen at -70°C. HIV-1 was quantified by p24 ELISA performed using the HIV-1 p24 Antigen EIA kit (Beckman Coulter). Virus concentration was obtained by loading equal amounts of the different virus on a 20% sucrose cushion and then centrifuging at 20,000Xg for 3 hours at 4°C C for experiments of Figure 1C or at 35,000Xg for 1.5 hours at 4°C for experiments of Figures 2B and 2C.
Infectivity analysis and western blot
For spreading infections experiments, 2×106 PHA stimulated PBMCs from two different donors were infected over-night with 50ng p24/well of R7/3 YU2 EGFP and its IN mutants in a 24 well plate, washed three times with PBS and re-fed with complete RPMI-1640 medium plus 40 units IL-2 /ml, supernatant’s p24 values were assessed at day 4, 6, 11, 13, 18, and 21 days post-infection.
TZM cells were obtained through the AIDS Research and Reference Program, Division of AIDS, NIAID, NIH, from Dr. J. Kappes.
For single round infections TZM cells were plated in 24 well plates at 40,000 cells/well, and infected with serial dilutions of the WT as well as of the IN mutant viruses. Infections were carried out overnight in the presence of 8μg/ml of polybrene. The second day after infection cells were trypsinized, fixed with PBS 3% formaldehyde (Tousimis) and fluorescence was analyzed by flow cytometry using FACScalibour (Becton Dickinson). 20,000 events were acquired per sample.
Integration was assessed by using an integration-defective R7/3 CMV EGFP virus carrying an IN with a substitution at position D116 and complemented with the pLR2P-R-PC-IN vector or its N-terminal mutants. We infected TZM cells and split them 1:10 at day 2, 6 and 10 post-infection in order to dilute the unintegrated viral cDNA. At the same time points, aliquots of the infected cells were analyzed by flow cytometry for EGFP expression.
Western blots were carried out by separating equal amounts of concentrated virions, lysed in 4X loading buffer, on a 10% polyacrylamide gel (NuPAGE, Invitrogen). Proteins were then transferred to a PVDF membrane (Immobilon, Millipore) and probed either with anti-IN monoclonal antibody 6G5 (Nilsen et al., 1996) or with an HIV-1 patient serum. Membranes were then incubated with horseradish peroxidase conjugated secondary antibodies, and developed with SuperSignal West Pico (Pierce) (anti-HIV-1) or SuperSignal West Femto (Pierce) (anti-IN).
Quantitative PCR
TZM cells plated at a density of 40,000 cells/well, were infected with 100ng/well of VSV-G pseudotyped R7/3 CMV EGFP reporter virus and its IN mutants previously treated with 150 units/ml of DNase I (Roche) for 30 min at 37°C. Infections were carried out for 5 hr in the presence of 8μg/ml of polybrene. After rinsing with PBS cells were fed with complete medium. Cells were harvested at time points 5, 10, 25 hr post-infection, pelleted and frozen at -70°C. Total genomic DNA was extracted using QIAamp DNA Blood kit (Qiagen). 2μl of each sample were used for qPCR. Primers used for the detection of late reverse transcription products were the following: primer GAG-3 CAGCATTATCAGAAGGAGCCAC and GAG-4 ATGTCACTTCCCCTTGGTTCTCT. SYBR green (Molecular Probes) was used as reporter fluorophore and reactions and acquisitions were performed using 7500 Real Time PCR System (Applied Biosystems). Normalization was carried out by measuring the DNA concentration of each sample using PicoGreen dsDNA Quantitation Reagent (Molecular Probes) following manufacturers instructions. Data are presented as number of late reverse transcription molecules per nanogram of total genomic DNA.
Acknowledgments
We thank Cecilia Cheng-Mayer, Paul Bieniasz, Ines Chen and David Ho for advice, support and critically reading the manuscript. We also like to thank Wendy Chen for her help with the figures. This work was supported by grants from the National Institutes of Health (NIH) number R21 AI056987 to L.C.F.M. and R01 AI064001 to V.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ao Z, Fowke KR, Cohen EA, Yao X. Contribution of the C-terminal trilysine regions of human immunodeficiency virus type 1 integrase for efficient reverse transcription and viral DNA nuclear import. Retrovirology. 2005;2:62. doi: 10.1186/1742-4690-2-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmair A, Finley D, Varshavsky A. In vivo half-life of a protein is a function of its amino-terminal residue. Science. 1986;234(4773):179–86. doi: 10.1126/science.3018930. [DOI] [PubMed] [Google Scholar]
- Bartel B, Wunning I, Varshavsky A. The recognition component of the N-end rule pathway. EMBO J. 1990;9(10):3179–89. doi: 10.1002/j.1460-2075.1990.tb07516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busschots K, Vercammen J, Emiliani S, Benarous R, Engelborghs Y, Christ F, Debyser Z. The interaction of LEDGF/p75 with integrase is lentiviral-specific and promotes DNA binding. J Biol Chem. 2005 doi: 10.1074/jbc.M411681200. [DOI] [PubMed] [Google Scholar]
- Cherepanov P, Maertens G, Proost P, Devreese B, Van Beeumen J, Engelborghs Y, De Clercq E, Debyser Z. HIV-1 integrase forms stable tetramers and associates with LEDGF/p75 protein in human cells. J Biol Chem. 2003;278(1):372–81. doi: 10.1074/jbc.M209278200. [DOI] [PubMed] [Google Scholar]
- Ciuffi A, Llano M, Poeschla E, Hoffmann C, Leipzig J, Shinn P, Ecker JR, Bushman F. A role for LEDGF/p75 in targeting HIV DNA integration. Nat Med. 2005;11(12):1287–9. doi: 10.1038/nm1329. [DOI] [PubMed] [Google Scholar]
- Dohmen RJ, Madura K, Bartel B, Varshavsky A. The N-end rule is mediated by the UBC2(RAD6) ubiquitin-conjugating enzyme. Proc Natl Acad Sci U S A. 1991;88(16):7351–5. doi: 10.1073/pnas.88.16.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durocher Y, Perret S, Kamen A. High-level and high-throughput recombinant protein production by transient transfection of suspension-growing human 293-EBNA1 cells. Nucleic Acids Res. 2002;30(2):E9. doi: 10.1093/nar/30.2.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman A. In vivo analysis of retroviral integrase structure and function. Adv Virus Res. 1999;52:411–26. doi: 10.1016/s0065-3527(08)60309-7. [DOI] [PubMed] [Google Scholar]
- Engelman A, Englund G, Orenstein JM, Martin MA, Craigie R. Multiple effects of mutations in human immunodeficiency virus type 1 integrase on viral replication. J Virol. 1995;69(5):2729–36. doi: 10.1128/jvi.69.5.2729-2736.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg MB, Jarrett RF, Aldovini A, Gallo RC, Wong-Staal F. HTLV-III expression and production involve complex regulation at the levels of splicing and translation of viral RNA. Cell. 1986;46(6):807–17. doi: 10.1016/0092-8674(86)90062-0. [DOI] [PubMed] [Google Scholar]
- Fletcher TM, 3rd, Soares MA, McPhearson S, Hui H, Wiskerchen M, Muesing MA, Shaw GM, Leavitt AD, Boeke JD, Hahn BH. Complementation of integrase function in HIV-1 virions. EMBO J. 1997;16(16):5123–38. doi: 10.1093/emboj/16.16.5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koken MH, Reynolds P, Jaspers-Dekker I, Prakash L, Prakash S, Bootsma D, Hoeijmakers JH. Structural and functional conservation of two human homologs of the yeast DNA repair gene RAD6. Proc Natl Acad Sci U S A. 1991;88(20):8865–9. doi: 10.1073/pnas.88.20.8865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano M, Vanegas M, Fregoso O, Saenz D, Chung S, Peretz M, Poeschla EM. LEDGF/p75 determines cellular trafficking of diverse lentiviral but not murine oncoretroviral integrase proteins and is a component of functional lentiviral preintegration complexes. J Virol. 2004;78(17):9524–37. doi: 10.1128/JVI.78.17.9524-9537.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd AG, Tateishi S, Bieniasz PD, Muesing MA, Yamaizumi M, Mulder LC. Effect of DNA repair protein Rad18 on viral infection. PLoS Pathog. 2006;2(5):e40. doi: 10.1371/journal.ppat.0020040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R, Ghory HZ, Engelman A. Genetic analyses of conserved residues in the carboxyl-terminal domain of human immunodeficiency virus type 1 integrase. J Virol. 2005;79(16):10356–68. doi: 10.1128/JVI.79.16.10356-10368.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R, Limon A, Ghory HZ, Engelman A. Genetic analyses of DNA-binding mutants in the catalytic core domain of human immunodeficiency virus type 1 integrase. J Virol. 2005a;79(4):2493–505. doi: 10.1128/JVI.79.4.2493-2505.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R, Vandegraaff N, Cherepanov P, Engelman A. Lys-34, dispensable for integrase catalysis, is required for preintegration complex function and human immunodeficiency virus type 1 replication. J Virol. 2005b;79(19):12584–91. doi: 10.1128/JVI.79.19.12584-12591.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madura K, Dohmen RJ, Varshavsky A. N-recognin/Ubc2 interactions in the N-end rule pathway. J Biol Chem. 1993;268(16):12046–54. [PubMed] [Google Scholar]
- Mulder LC, Chakrabarti LA, Muesing MA. Interaction of HIV-1 integrase with DNA repair protein hRad18. J Biol Chem. 2002;277(30):27489–93. doi: 10.1074/jbc.M203061200. [DOI] [PubMed] [Google Scholar]
- Mulder LC, Muesing MA. Degradation of HIV-1 integrase by the N-end rule pathway. J Biol Chem. 2000;275(38):29749–53. doi: 10.1074/jbc.M004670200. [DOI] [PubMed] [Google Scholar]
- Nilsen BM, Haugan IR, Berg K, Olsen L, Brown PO, Helland DE. Monoclonal antibodies against human immunodeficiency virus type 1 integrase: epitope mapping and differential effects on integrase activities in vitro. J Virol. 1996;70(3):1580–7. doi: 10.1128/jvi.70.3.1580-1587.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S, Pursley MH, Grandgenett DP. Efficient Concerted Integration by Recombinant Human Immunodeficiency Virus Type 1 Integrase without Cellular or Viral Cofactors. J Virol. 2002;76(7):3105–3113. doi: 10.1128/JVI.76.7.3105-3113.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasaki T, Mulder LC, Iwamatsu A, Lee MJ, Davydov IV, Varshavsky A, Muesing M, Kwon YT. A family of mammalian E3 ubiquitin ligases that contain the UBR box motif and recognize N-degrons. Mol Cell Biol. 2005;25(16):7120–36. doi: 10.1128/MCB.25.16.7120-7136.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasara T, Maga G, Hottiger MO, Hubscher U. HIV-1 reverse transcriptase and integrase enzymes physically interact and inhibit each other. FEBS Lett. 2001;507(1):39–44. doi: 10.1016/s0014-5793(01)02945-3. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove L, Christ F, Van Maele B, De Rijck J, Gijsbers R, Van den Haute C, Witvrouw M, Debyser Z. Transient and stable knockdown of the integrase cofactor LEDGF/p75 reveals its role in the replication cycle of human immunodeficiency virus. J Virol. 2006;80(4):1886–96. doi: 10.1128/JVI.80.4.1886-1896.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanegas M, Llano M, Delgado S, Thompson D, Peretz M, Poeschla E. Identification of the LEDGF/p75 HIV-1 integrase-interaction domain and NLS reveals NLS-independent chromatin tethering. J Cell Sci. 2005;118(Pt 8):1733–43. doi: 10.1242/jcs.02299. [DOI] [PubMed] [Google Scholar]
- Varshavsky A. The ubiquitin system. Trends Biochem Sci. 1997;22(10):383–7. doi: 10.1016/s0968-0004(97)01122-5. [DOI] [PubMed] [Google Scholar]
- Wiskerchen M, Muesing MA. Human immunodeficiency virus type 1 integrase: effects of mutations on viral ability to integrate, direct viral gene expression from unintegrated viral DNA templates, and sustain viral propagation in primary cells. J Virol. 1995;69(1):376–86. doi: 10.1128/jvi.69.1.376-386.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Liu H, Xiao H, Conway JA, Hehl E, Kalpana GV, Prasad V, Kappes JC. Human immunodeficiency virus type 1 integrase protein promotes reverse transcription through specific interactions with the nucleoprotein reverse transcription complex. J Virol. 1999;73(3):2126–35. doi: 10.1128/jvi.73.3.2126-2135.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee JK, Friedmann T, Burns JC. Generation of high-titer pseudotyped retroviral vectors with very broad host range. Methods Cell Biol. 1994;43(Pt A):99–112. doi: 10.1016/s0091-679x(08)60600-7. [DOI] [PubMed] [Google Scholar]
- Yoder KE, Bushman FD. Repair of gaps in retroviral DNA integration intermediates. J Virol. 2000;74(23):11191–200. doi: 10.1128/jvi.74.23.11191-11200.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]