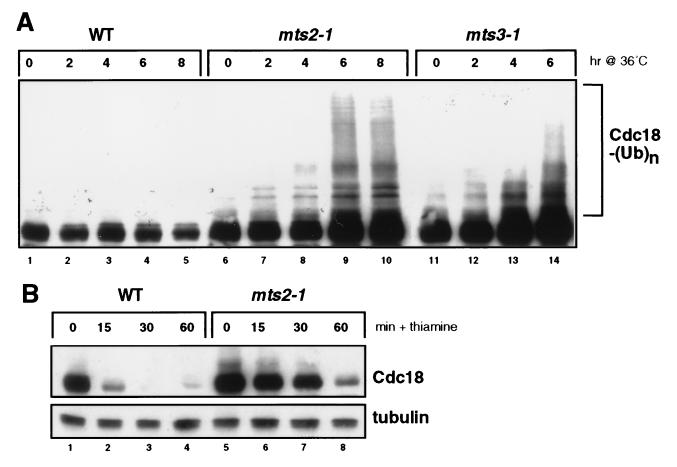
Figure 1.
Degradation of the Cdc18 replication initiator protein is mediated by the ubiquitin-proteasome pathway in fission yeast. (A) A hemagglutinin (HA) epitope-tagged form of Cdc18 was expressed from the weak thiamine-repressible nmt1 promoter (REP41X) in a wild-type strain (lanes 1–5) or in two temperature-sensitive (ts) proteasome mutants (mts2–1, lanes 6–10; mts3–1, lanes 11–14). Logarithmically growing cells were shifted from 25°C to 36°C, and the abundance and electrophoretic mobility of Cdc18 assessed by immunoblotting with anti-HA mAb 12CA5. The position of the Cdc18-multiubiquitin adducts is indicated. (B) The half-life of Cdc18 in wild-type and mts2–1 strains was measured by shifting cells to 36°C for 3 hr (to inactivate the mutant proteasomes) and then adding thiamine to acutely repress de novo expression from the nmt1 promoter. Cdc18 degradation was monitored by immunoblotting with 12CA5 mAb. The same membrane was blotted with anti-tubulin mAb B512 to confirm equal loading.