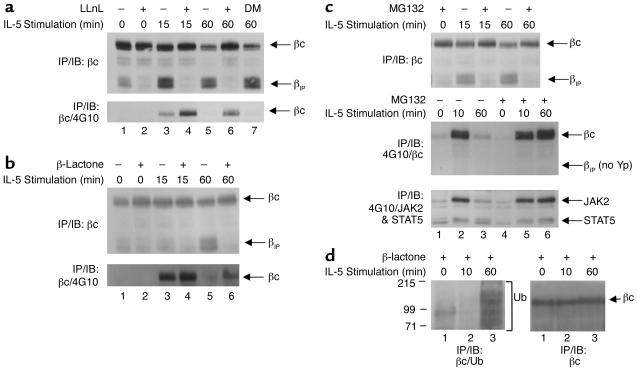
Figure 2.
IL-5 induces proteasomal degradation of the βc cytoplasmic domain. (a) TF1 cells that were cytokine-starved for 24 hours were left untreated (lanes 1, 3, and 5) or were pretreated with 50 μM LLnL (lanes 2, 4, and 6) or 0.01% (vol) DMSO (lane 7) for 1 hour prior to IL-5 stimulation (10 ng/ml) (top and bottom panels). Whole-cell lysates were immunoprecipitated with anti-βc monoclonal antibody S-16, and immunoblotted with anti-βc polyclonal antibodies (top panel). The top arrow indicates full-length βc receptors; the bottom arrow corresponds to βIP. The membrane was stripped and reprobed with monoclonal antibody 4G10 (bottom panel). (b) Same as in a except the cells were pretreated with 20 μM β-lactone for 1 hour, followed by IL-5 stimulation. The top panel shows analysis by IP/IB with the same anti-βc antibodies described in a. The membrane was then stripped and reprobed with 4G10 (bottom panel). (c) Same as in a and b, except that the cells were pretreated with 50 μM MG132 for 1 hour, followed by IL-5 stimulation (all panels). The top panel was analyzed by IP/IB with the same anti-βc antibodies used in a and b. The middle panel was immunoprecipitated with 4G10, and immunoblotted with the anti-βc polyclonal antibodies. The blot was then stripped and reprobed with both anti-JAK2 and anti-STAT5 antibodies (bottom panel). (d) TF1 cells (3 × 107 cells/IP) that had been cytokine-starved for 24 hours were pretreated with 30 μM β-lactone for 1 hour, followed by IL-5 stimulation (15 ng/ml). Whole-cell lysates were immunoprecipitated with anti-βc antibodies and immunoblotted with anti-ubiquitin monoclonal antibodies (left panel). The bracket designates βc ubiquitination, as well as other ubiquitinated proteins that coimmunoprecipitated with anti-βc antibodies. The blot in the left panel was stripped and reprobed with anti-βc antibodies (right panel). DM, DMSO, Yp, tyrosine phosphorylation; Ub, ubiquitin.