Abstract
OBJECTIVE:
To determine whether influenza immunization is associated with early side effects, a deleterious impact on the illness course and depressed antibody response in patients with chronic fatigue syndrome (CFS).
DESIGN:
Prospective, randomized, double-blind, placebo controlled trial. CFS patients and healthy volunteers filled out a questionnaire on immunization side effects and had hemagglutination-inhibiting (HI) antibody titres measured pre- and three weeks after immunization. CFS patients completed symptom and function questionnaires before and during the six-week, postimmunization period.
SETTING:
Ambulatory care.
POPULATION STUDIED:
Convenience sample of 40 CFS patients fulfilling the Centers for Disease Control and Prevention criteria and 21 demographically matched healthy volunteers.
INTERVENTIONS:
CFS patients were randomly selected to receive commercially available whole virus influenza vaccine (n=19) or an injection of saline placebo (n=21). Healthy volunteers received vaccine only.
MAIN RESULTS:
As a group, immunized CFS patients had lower geometric mean HI antibody rises than healthy volunteers (P<0.001). However, there was no difference in the rates of fourfold titre rises, and immunization did achieve a probably protective titre (1:32 or greater) in most CFS patients. No difference could be detected between immunized and placebo CFS patients in immunization side effects, although CFS patients as a group reported four times as many side effects as healthy volunteers. Further, in the six weeks following immunization, placebo and immunized CFS patients did not demonstrate any differences in terms of functioning, symptom severity and sleep disturbance.
CONCLUSIONS:
In patients with CFS, influenza immunization is safe, not associated with any excess early reactions, and stimulates an immunizing response comparable with that of healthy volunteers.
Key Words: Chronic fatigue syndrome, Humoral immunization response, Influenza immunization
BACKGROUND
Chronic fatigue syndrome (CFS), also known as 'myalgic encephalomyelitis' (ME), is a disorder characterized by the new onset of a persistent or relapsing fatigue that fails to resolve with bedrest and that significantly impairs daily activity for six months or longer. Conditions that may account for the patient's symptoms must be excluded before CFS may be diagnosed (1). Three definitions of CFS exist in the literature, but all include the preceding description of debilitating fatigue. The Centers for Disease Control and Prevention (CDC, Atlanta, Georgia) definition stipulates that patients must also experience at least six symptoms and two signs, or eight symptoms of CFS. Signs and symptoms include fever, pharyngitis, cervical adenopathy, myalgias, postexertional fatigue, headaches, neuropsychological disturbances and sleep disorder (1). The Australian definition does not require that patients experience any particular sign or symptom but specifies the presence of neuropsychiatric impairment and/or abnormal cell-mediated immunity (2). The Oxford definition is the most lenient, requiring only the existence of debilitating fatigue (3).
In a community-based population, the point prevalence of CFS was reported to be 98 to 267 cases/100,000 (4). CFS sufferers are often unable to work and are dependent on disability insurance from private insurers and government pension plans. Conditions that seem to exacerbate the illness are any undue physical or mental stress, such as an acute viral illness or psychologically stressful event. These may result in a setback that may last several months, further compounding the disability.
The etiology of CFS continues to be the subject of vigorous debate. CFS has been attributed to the reactivation of latent infectious agents and/or immune dysfunction (although evidence suggests only in vitro immune alterations) (5-9). The high rate of pre- and comorbid psychiatric illnesses in CFS patients has prompted some theorists to advance a psychological basis for the disorder (10-11). Demitrack (12) suggested that the phenomenological overlap between CFS and primary psychiatric illnesses reflects the existence of a shared, final common biological pathway, the hypothalamic-pituitary-adrenal axis (HPA), which may become disturbed by a variety of infectious or noninfectious pathophysiological antecedents.
In our experience, some patients are reluctant to receive common preventive agents such as vaccines for fear of exacerbating CFS symptoms. To provide objective data on immunization in CFS patients, we investigated the effect of commercially available influenza vaccine on the following: the specific antibody response; the rate of early post-immunization side effects; and any effects of the immunization on the clinical course of CFS for the following six weeks. Our null hypothesis was that there would be no difference in antibody responses for CFS vaccinees compared with healthy vaccinees and no difference in the clinical course of patients with CFS who received vaccine compared with CFS patients receiving the placebo immunization.
PATIENTS AND METHODS
Patients:
Because of the difficulty in demonstrating a fourfold antibody rise in individuals with higher pre-existing titres, prospective participants who had received an influenza immunization within two years of study entry were excluded. Individuals who were allergic to eggs were also excluded. The appropriate ethics committee approval was obtained, and participants provided signed, informed consent. Two samples were recruited: CFS patients who were randomly selected to receive immunization (n=19) or placebo (n=21); and healthy volunteers (n=21).
CFS group:
Outpatients who attended the CFS clinic were eligible to participate if they were diagnosed with CFS as defined by CDC criteria (1). A total of 107 patients were contacted by telephone. Fifty-two patients refused to participate or were deemed ineligible. Of the remaining 55 patients, 15 were excluded after signing informed consent. This group included eight patients who met the criteria for psychiatric exclusion, six patients were unable to make repeated visits to the clinic, and one patient was diagnosed with asthma as a cause of chronic fatigue. No differences were found between ineligible or excluded patients and the final CFS sample regarding the following demographics: age (P=0.58; t-test), sex ratio (P=0.77; χ2) and duration of CFS symptoms (P=0.71; t-test).
Control group:
Twenty-five age (within three years) and sex-matched healthy volunteers who responded to advertisements published in various institutional newsletters were recruited for the comparative assessment of antibody response and immediate untoward effects of influenza vaccination. Twenty-one returned for the three week, postimmunization blood sampling. These persons were employees at the Vancouver Hospital, the University of British Columbia and Simon Fraser University, Vancouver, British Columbia.
Methods:
CFS group:
All potential CFS patients underwent a history and physical examination. After demographic data and information on concomitant medication use were collected, a psychiatric assessment was performed using the Beck Depression Inventory (BDI) (13) and the Computerized Diagnostic Interview Schedule (CDIS) (14). After reviewing the CDIS responses, a psychiatrist interviewed patients to verify data generated by the CDIS.
To assess the effects of vaccination, data were collected on pre- and postimmunization health status and CFS symptoms. In addition, all patients maintained a seven-day diary of injection-related side effects during the week following immunization. All CFS patients returned self-administered questionnaires weekly by mail, using prestamped, addressed envelopes. Due to the cognitive impairment associated with CFS, participants were given a nine-week calendar identifying office visit dates and when each questionnaire was to be completed. Questionnaires were staggered throughout the study to reduce fatigue and measurement error. Despite the heavy time and concentration burden imposed by the questionnaires, patients completed the following questionnaires with few omissions:
The Sickness Impact Profile (SIP) (15) is a 136-item self-report questionnaire that measures sickness-related physical and psychosocial dysfunction experienced by respondents in the preceding three months. Patients completed the SIP preimmunization and at three and six weeks postimmunization.
The Symptom Check List 90 (SCL-90) (16) is a 90-item instrument that assesses the number and severity of psychological and physical symptoms. The SCL-90 was completed before immunization and weekly for six weeks postimmunization.
The BDI (13) is a reliable and valid scale that describes 21 symptoms of depression. Subjects were instructed to fill out the BDI preimmunization and weekly for six weeks after immunization.
The Visual Analogue Scale for Fatigue (VAS-F) (17) uses an 18-item, nonsegmented analogue scale to measure fatigue and energy. Reliability for the fatigue and energy subscales in a group of sleep-disordered patients was calculated as 0.96 and 0.95, respectively (17). The instrument has demonstrated excellent concurrent validity (17). The VAS-F was administered for seven consecutive days preimmunization, seven consecutive days postimmunization, and then once a week for four weeks.
The St Mary's Hospital Sleep Questionnaire (SMHSQ) (18) is a 14-item instrument that evaluates a respondent's sleep and early morning behaviour for the preceding 24 h. Using Kendall's tau, test-retest reliability for the items ranged between 0.70 and 0.96 (18). Participants completed the SMHSQ each day for one week before and immediately following immunization, then each day for one week for weeks three and six after immunization.
The CFS-related Symptom Time/Severity Questionnaire (CFS-SS) was developed by the authors to monitor patients' progress in the CFS clinic. Using a Likert scale, the CFS-SS provided a comparative semiquantitative measure of maximum symptom severity and duration between the two CFS treatment groups. The CFS-SS was completed weekly for three weeks before immunization, then weekly for six weeks after immunization.
To measure early vaccine side effects, the Vaccine Evaluation Centre Questionnaire (VECQ) was used. Patients were given a thermometer and asked to measure their oral temperature four times daily for seven days postimmunization. They were also asked to record any immunization-related symptoms and/or localized skin reactions each day.
Immunization:
CFS subjects were assigned to vaccination or placebo groups in a double-blind, randomized fashion. A computer generated program (PC PLAN, Gerard Dallal, United States), was used by the hospital pharmacy department to develop the randomization schedule. Only the pharmacist had access to the code. The investigator and all other study personnel were blinded. The pharmacy dispensed preloaded syringes labelled with each subject's initials and identification number. The research team injected CFS patients with either 0.5 mL normal saline or 0.5 mL of Fluviral S/F (IAF BioVac Inc, Canada), which is a whole virus vaccine containing antigens of A/Texas/36/91(H1N1), A/Beijing/32/92(H3N2) and B/Panama/45/90, recommended for the 1993-94 influenza season by the British Columbia Centre for Disease Control, Vancouver, British Columbia. Each injection was given intramuscularly into the deltoid using a 23 gauge needle, 2.5 cm in length.
Serum sampling:
Immediately before immunization and three weeks after immunization, a 10 mL specimen of blood was collected from each patient and allowed to clot. Sera were tested for the presence of hemagglutination-inhibiting (HI) antibodies against the homologous antigens using a standard protocol, except nonspecific inhibitors were removed by kaolin treatment of sera rather than receptor-destroying enzyme neuraminidase (19). All sera were tested in pairs, and any twofold rises in antibody were retested twice, and the 'two of three' concurrent values applied in the analysis of data. No other measurements of immunological function were made.
Control group:
Blood was obtained for antibody testing and all participants were immunized with 0.5 mL of Fluviral S/F (as described above) and instructed to complete the VECQ and record their temperatures four times a day for one week. Participants returned to the hospital to have blood drawn for antibody testing three weeks postimmunization. Laboratory analysis of the serum was identical to the procedure used for the CFS group.
Statistical analyses:
Baseline antibody titres were compared using the Mann Whitney U test. Comparison of the numbers of patients with fourfold titre rises were analyzed with the χ2 test, and magnitude of titre rise was analyzed using the two sample t-test.
Temperature data derived from the VECQ forms were expected to be normally distributed and were analyzed using ANOVA. Symptom scores were non-normally distributed, and the Kruskal Wallis one-way ANOVA and Mann Whitney U tests were used to compare the values between the groups. Analysis of covariance was used to analyze the function and severity questionnaire data. The postinjection mean level was compared between the vaccine and placebo groups while adjusting for the pretest mean.
Using Cohen's power tables, it was calculated that, in terms of overall group differences and group profiles, the study could detect a large effect size with a power of 0.70 (20). The effect size is the relative difference in the means between two comparisons divided by the standard deviation. A large effect size suggests a clinically significant difference and is demonstrated when the scores of two populations are highly divergent.
RESULTS
Sample:
The CFS sample was almost exclusively Caucasian (97%), and mostly composed of women (76%). Patients' ages ranged from 18 to 56 years, with a mean ± SD of 39±10. On average, patients had experienced CFS symptoms for 47±29 months. The mean age of illness onset was 36 years. These demographics are comparable with those reported by Ho-Yen and McNamara (21) in an analysis of data from several self-referral studies. Although statistically equivalent in age (P=0.13) and illness duration (P=0.63), the CFS treatment groups differed in medication use. Ten CFS vaccinees reported taking antidepressants versus four patients in the CFS placebo group. As well, five CFS vaccinees used sedatives, while only two patients in the CFS placebo group reported using sedation.
The healthy volunteers and the CFS vaccinees were well matched demographically. The average age of the controls and CFS vaccinees was 40 years, and no significant differences were found when the ages of the men (P=0.09, t-test) and women (P=0.47, t-test) in the CFS and control groups were compared. No individuals in either CFS group or the healthy volunteer group were taking, or had recently taken, immunosuppressive medication.
Antibody response to vaccination:
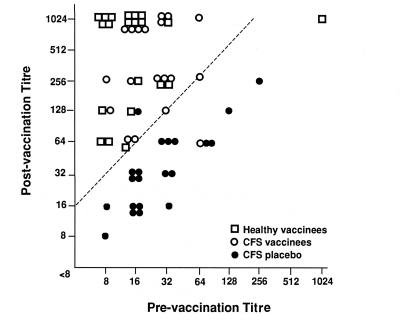
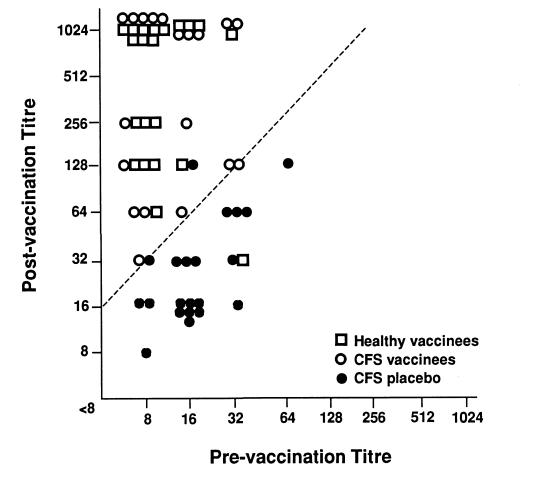
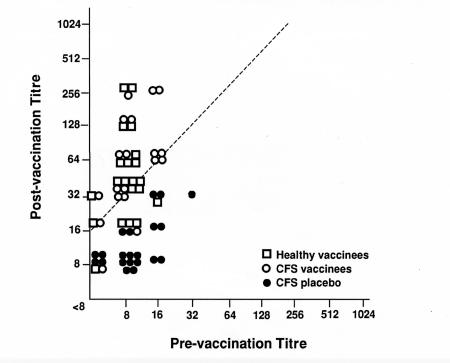
Figure 1,Figure 2 and Figure 3 outline the HI antibody response comparing the CFS vaccination, CFS placebo and the healthy volunteer groups for the vaccine antigens A/Texas/36/91(H1N1), A/Beijing/32/92(H3N2) and B/Panama/45/90. There was no statistical difference in patients with fourfold titre rise between the CFS vaccination and the healthy volunteer groups three weeks after vaccination. Curiously, the antibody response to B/Panama was not as robust as the influenza A antigens, but both healthy volunteers and CFS vaccinees showed this lessened response. Against A/Texas, 18 of 19 CFS vaccination, 20 of 21 healthy volunteers, and one of 21 in the CFS placebo group developed a fourfold rise in antibodies. To A/Beijing, 19 of 19 CFS vaccination, 20 of 21 healthy volunteers, and two of 21 CFS placebo groups had fourfold rises. Against B/Panama, 17 of 19, 16 of 21 and none of 21 individuals developed fourfold rises in the CFS vaccination, healthy volunteer and CFS placebo groups, respectively. The three placebo patients who developed fourfold rises were almost certainly infected with a wild type strain, and, therefore, it must be assumed that the antibody response for a similar proportion of the vaccinees may have been due to wild type incidental infection. However, the review of symptoms in the three groups failed to uncover any patients with a typical influenza illness during the postimmunization period.
Figure 1.
Reciprocal hemagglutination-inhibiting antibody titres against A/Texas/36/91 (H1N1) of healthy vaccinees, chronic fatigure syndrome (CFS) vaccinees, receiving influenza vaccine, and placebo-immunized CFS patients preimmunization and three weeks postimmunization. Slash line indicates fourfold rise
Figure 2.
Reciprocal hemagglutination-inhibiting antibody titres against A/Beijing/32/92 (H3N2) of healthy vaccinees, chronic fatigure syndrome (CFS) vaccinees, receiving influenza vaccine, and placebo-immunized CFS patients preimmunization and three weeks postimmunization. Slash line indicates fourfold rise
Figure 3.
Reciprocal hemagglutination-inhibiting antibody titres against B/Panama/45/90 preimmunization and three weeks postimmunization of healthy vaccinees, chronic fatigure syndrome (CFS) vaccinees and placebo-immunized CFS patients. Slash line indicates fourfold rise
The baseline prevaccination reciprocal geometric mean titres (GMT) for each vaccine antigen comparing the two groups of vaccinees, healthy versus CFS were: 13.5 versus 23.9 for A/Texas/H1N1 (P=0.007, Mann Whitney test), 10.4 versus 12.9 for A/Beijing/H3N2 (not significantly different), and 9.1 versus 11.9 for B/Panama (not significantly different).
There was a significantly greater magnitude of antibody titre rise in the healthy vaccinees compared with the CFS vaccinees except for B/Panama. The increase in GMT ± SD comparing healthy controls and CFS vaccinees was 33.1±2.1 and 14.3±1.86 (P<0.001, two sample t-test), respectively, for A/Texas/H1N1; 39.4±2.8 versus 26.70±2.5 (P<0.001) for A/Beijing/H3N2; and 5.6±2.1 versus 5.9±0.03 for B/Panama.
Immediate side effects from vaccination:
Comparison of the postimmunization symptom scores for the three groups (Table 1) revealed a striking and statistically significant increase in the values for the 25th, 50th, and 75th percentiles for the CFS groups over the healthy volunteers group. However, there was no statistical difference in immediate side effects between CFS patients who received influenza vaccine and those who received a saline injection. Similarly, there was no significant difference in the average highest daily temperature, an objective measurement, among the three groups.
TABLE 1.
Seven-day postimmunization symptom scores comparing chronic fatigue syndrome (CFS) vaccines, placebo-immunized CFS patients and healthy vaccines
| Symptom scores for percentiles of groups | ||||
|---|---|---|---|---|
| Group | 25th | 50th | 75th | P |
| CFS vaccination | 3.1 | 4.1 | 6.6 | |
| CFS placebo | 1.9 | 3.4 | 5.0 | <0.0001* |
| Healthy volunteers | 0.3 | 0.7 | 2.1 | |
Mann Whitney U test
Effects of vaccination on the course of CFS:
Patients completed a battery of questionnaires to measure the effects of vaccination on the course of CFS. Using each patient as his or her own control, comparisons of the preimmunization mean values with the mean values recorded over the six weeks after injection revealed that only the SMHSQ demonstrated statistically significant differences between the CFS vaccinees and placebo groups, with the placebo group experiencing higher scores for sleep latency (length of time to fall asleep at night). However, this finding likely reflects differences that were shown to exist between the two groups at baseline and may be attributable to the increased use of antidepressants and sedatives in the CFS vaccination group.
DISCUSSION
This study is the first to examine the effect of immunization on the function of patients with CFS in a double-blind protocol. Influenza immunization appears to be safe, with no more untoward early side effects than a placebo injection. However, CFS patients in both the placebo and immunized groups reported a number of constitutional symptoms in the first week postimmunization. Because the VECQ measures immunization-related effects, such as malaise and muscle aches, which are also associated with CFS, it is difficult to determine whether these symptoms could be attributed to immunization or CFS. Because there were no significant differences between the CFS placebo and vaccine groups in terms of symptom scores, we concluded that few if any of the postimmunization complaints in the CFS vaccinees were caused by the vaccine. This is supported further by the lack of any difference in highest daily mean temperature for the week.
The immunizing response to the three vaccine antigens studied was normal in patients with CFS. It has been suggested, based on laboratory studies (6-9), that CFS is a condition of immune unresponsiveness or dysregulation. This has led to the term chronic fatigue immune dysfunction syndrome (CFIDS). Our data indicate a blunted humoral immune response to the influenza A antigens and less so to B/Panama. However, most of the CFS vaccinees mustered a fourfold HI antibody rise to at least a titre of 1:32, which has been correlated with protection against wild type infection (22,23).
It is unlikely that the blunted responses of the CFS vaccinees were related to antidepressant use. Selective serotonin-reuptake inhibitors were the preferred antidepressant agents used by the CFS vaccinees, and the selective serotonin-reuptake inhibitor, fluoxetine, has been found to normalize levels of cytotoxic T cells and natural killer cell cytoxicity in a group of CFS patients treated for eight weeks in an uncontrolled trial (24).
In a previous study, differences were found in the cellular and humoral responses of 20 CFS patients fulfilling CDC criteria compared with 20 age- and sex-matched controls (8). CFS patients demonstrated differences in cell-mediated immunity, including a decreased response to soluble antigens. The humoral antibody titre response at two weeks postimmunization to a primary pneumococcal polysaccharide antigen was reported to be decreased in six of 20 CFS patients, but the response in the controls was not reported for comparison. The antibody response to diphtheria and tetanus protein antigens (anamnestic response to a booster dose of diphtheria-tenanus) was reportedly normal, although data in patient and control groups were not shown.
Stress may account for differences in immune functioning between CFS patients and healthy people. Studies have suggested that CFS patients are vulnerable to stress. Researchers found that CFS patients experienced more loss-related life events 12 months before CFS onset than healthy control groups (25), attributed the onset of the disorder to stressful events and/or lifestyles (26), and linked exacerbations of CFS symptoms to stressful events (10,12,27). In addition, CFS has been shown to compromise social functioning and reduce patients' social support networks (28,29).
In two samples, Glaser et al (30) demonstrated differences in cellular and humoral immune responses to vaccines, which the authors attributed to stress. In an older population consisting of caregivers of Alzheimer's patients, subjects inoculated with commercially available, inactive influenza vaccine showed a poorer antibody response and more rapid decline in the virus-specific T cell response over time than matched controls. Similar findings were reported in a group of medical students who were inoculated with hepatitis B vaccine injections following a three-day examination period. Twelve of the 48 medical students seroconverted after the first injection. These students were found to fall into the lower stress/low anxiety group. In addition, students who reported greater social support and lower anxiety and stress demonstrated a higher antibody response to the vaccine and a more vigorous T cell response to hepatitis B surface antigen. A variety of mechanisms have been hypothesized to account for the effect of stress on the immune system (31,32).
We observed two patients in the placebo group with fourfold rises in antibody to A/Beijing/H3N2 and one with a fourfold rise to A/TexasH11. Because the study was conducted in the fall of 1993, and both H3N2 and H11 wild type viruses were circulating in the community near the end of the testing period, the placebo patients likely acquired a serological rise in response to a wild type infection. Although we had hoped to avoid this by beginning the study in the early fall, any 'contaminating' effect of wild virus infection in the study population appeared to be small and, at any rate, would have assumedly affected each of the vaccine groups equally.
At the beginning of this study we had some difficulty in recruiting CFS patient volunteers, the most common reason for refusal being fear of some adverse effect of the vaccine on their illness. However, based on this sample, influenza vaccination has no demonstrable adverse effect on CFS. Although the patient population studied was small, and thus the chance of type 2 error large, many sequential measurements were made on the same patient, who served as his or her own control. The study was statistically sound for 'large differences', a term coined by Cohen (20) for differences that should be clinically recognizable. It is likely, although not proven, that similar results to ours would occur with other influenza vaccine antigen preparations. Thus, we feel that physicians who choose to immunize CFS patients against influenza can do so with the expectation that immunization will be effective and not have a deleterious impact on the course of the disorder.
Acknowledgments
The authors are grateful for the assistance of Mr Darryl Cook for laboratory advice, Dr Jonathan Berkowitz for statistical assistance, Dr David Scheifele of the Vaccine Evaluation Centre and Mrs Maureen Osborne for manuscript preparation. This study was supported by Grant 6610-2000-54 from the Health Canada National Health Research Development Program. Writing of this paper was facilitated by funding to Kenna Sleigh from the British Columbia Health Research Foundation (Studentship Grant #51[99]), and the British Columbia Medical Services Foundation (Predoctoral Fellowship).
References
- 1.Schluederberg A, Straus SE, Peterson P, et al. NIH conference. Chronic fatigue syndrome research. Definition and medical outcome assessment.Ann Intern Med 1992;15:325-31. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd AR, Wakefield D, Boughton C, Dwyer J. What is myalgic encepalomyelitis?Lancet 1988;i:1286-7. [DOI] [PubMed] [Google Scholar]
- 3.Sharpe MC, Arnold LC, Banatvala JE, et al. A report -- Chronic fatigue syndrome: Guidelines for research.J Roy Soc Med 1991;84:118-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchwald D, Umali P, Umali J, Kith P, Pearlman T, Komaroff AL. Chronic fatigue and the chronic fatigue syndrome: Prevalence in a Pacific Northwest health care system.Ann Intern Med 1995;123:81-8. [DOI] [PubMed] [Google Scholar]
- 5.Landay AL, Jessop C, Lennette ET, Levy JA. Chronic fatigue syndrome: clinical condition associated with immune activation.Lancet 1991;338:707-12. [DOI] [PubMed] [Google Scholar]
- 6.Chao CC, Janoff EN, Hu SX, et al. Altered cytokine release in peripheral blood mononuclear cell cultures from patients with chronic fatigue syndrome.Cytokine 1991;3:292-8. [DOI] [PubMed] [Google Scholar]
- 7.Klimas NG, Salvato FR, Morgan R, Fletcher MA. Immunologic abnormalities in chronic fatigue syndrome.J Clin Microbiol 1990;28:1403-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta S, Vayuvegula B. A comprehensive immunologic analysis in chronic fatigue syndrome.Scand J Immunol 1991;33:319-27. [DOI] [PubMed] [Google Scholar]
- 9.Mawle AC, Nisenbaum R, Dobbins JG, et al. Immune responses associated with chronic fatigue syndrome: A case-control study.J Infect Dis 1997;175:136-41. [DOI] [PubMed] [Google Scholar]
- 10.Wessely S, Butler S, Chadler T, David A. The cognitive behavioral management of post-viral fatigue syndrome. In: Jennings R, Mowbray RJ, eds. Post-viral Fatigue Syndrome.Chichester: Wiley,, 1991:305-34. [Google Scholar]
- 11.Manu P, Matthews D, Lane T. The mental health of patients with a chief complaint of chronic fatigue. A prospective evaluation and follow-up.Arch Intern Med 1988;148:2213-7. [PubMed] [Google Scholar]
- 12.Demitrack MA. Chronic fatigue syndrome: A disease of the hypothalamic-pituitary-adrenal axis?Ann Med 1994;26:1-5. [DOI] [PubMed] [Google Scholar]
- 13.Beck A, Ward C, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression.Arch Gen Psychiatry 1961;4:561-71. [DOI] [PubMed] [Google Scholar]
- 14.Robins L, Helzer J. The Computerized Diagnostic Interview Schedule.Washington: National Institute of Mental Health, 1991. [Google Scholar]
- 15.Bergner M, Bobbitt R, Kressel S, Pollard W, Gilson B, Morris J. The Sickness Impact Profile: conceptual formulation and methodology for the development of a health status measure.Int J Health Serv 1976;6:393-410. [DOI] [PubMed] [Google Scholar]
- 16.Derogatis L. SCL-90 Administration, Scoring and Procedures: Manual-I for the Revised Version and Other instruments of the psychopathology Rating Scale Series. Baltimore: University School of Medicine, 1977. [Google Scholar]
- 17.Lee K, Hicks G, Nino-Murcia G. Validity and reliability of a scale to assess fatigue.Psychiatry Res 1991;36:291-8. [DOI] [PubMed] [Google Scholar]
- 18.Ellis B, Johns M, Lancaster R, Raptopoulos P, Angelopoulos N, Priest R. The St Mary's Hospital Sleep Questionnaire: a study of reliability.Sleep 1981;4:93-7. [DOI] [PubMed] [Google Scholar]
- 19.Robinson RQ, Dowdle WR. Influenza viruses. In: Lennette EH, Schmidt NJ, eds. Diagnostic Procedures for Viral and Rickettsial Infections, 4th edn.New York: American Public Health Association Inc, 1969:414-33. [Google Scholar]
- 20.Cohen J. Statistical Power Analysis for the Behavioral Sciences, 2nd edn. Hillsdale: L Erlbaum Associates, 1988. [Google Scholar]
- 21.Ho-Yen DO, McNamara I. General practitioners' experience of the chronic fatigue syndrome.Br J Gen Pract 1991;41:324-6. [PMC free article] [PubMed] [Google Scholar]
- 22.Stiver HG, Graves P, Eickhoff TC, Meiklejohn G. Efficacy of "Hong Kong" vaccine in preventing "England" variant influenza in 1972.N Engl J Med 1973;289:1267-71. [DOI] [PubMed] [Google Scholar]
- 23.Hobson D, Curry RL, Beare AS. Hemagglutination-inhibiting antibody titres as a measure of protection against influenza in man. In: Perkins FT, Regamy RH, eds. International Symposium on Influenza Vaccines for Men and Horses, London, 1972. Symposium Series of Immunological Standardization, No 2.New York: Karger, 1973:164-8. [Google Scholar]
- 24.Klimas N, Morgan R, Van Riel F, Fletcher MA. Observations regarding use of an antidepressant, fluoxetine, in chronic fatigue syndrome. In: Goodnick PJ, Klimas NG, eds. Chronic fatigue and Related Immune Deficiency Syndromes.Washington: American Psychiatric Press, 1993:95-108. [Google Scholar]
- 25.Stricklin A, Sewell M, Austad C. Objective measurement of personality variables in epidemic neuromyasthenia patients.S Afr Med J 1990;77:31-4. [PubMed] [Google Scholar]
- 26.Ware NC. Society, mind, and body in chronic fatigue syndrome: an anthropological view. In: Bock GR, Whelan J, eds. Chronic Fatigue Syndrome.Chichester: Wiley, 1993:62-82. [DOI] [PubMed] [Google Scholar]
- 27.Lutgendorf SK, Antoni MH, Ironson G, et al. Physical symptoms of chronic fatigue syndrome are exacerbated by the stress of Hurricane Andrew.Psychosom Med 1995:57:310-23. [DOI] [PubMed] [Google Scholar]
- 28.Schweitzer R, Kelly B, Foran A, Terry D, Whiting J. Quality of life in chronic fatigue syndrome.Soc Sci Med 1995;41:1367-72. [DOI] [PubMed] [Google Scholar]
- 29.Anderson JS, Ferrans CE. The quality of life of persons with chronic fatigue syndrome.J Nerv Ment Dis 1997;185:359-67. [DOI] [PubMed] [Google Scholar]
- 30.Glaser R, Kiecolt-Glaser JK, Malarkey WB, Sheridan JF. The influence of psychological stress on the immune response to vaccines.Ann N Y Acad Sci 1998;840:649-55. [DOI] [PubMed] [Google Scholar]
- 31.Demitrack M. Neuroendocrine research strategies in chronic fatigue syndrome. In: Goodnick PJ, Klimas NG, eds. Chronic Fatigue and Related Immune Deficiency Syndromes.Washington: American Psychiatric Press,1993:45-66. [Google Scholar]
- 32.Kaplan HB. Social psychology of the immune system: A conceptual framework and review of the literature.Soc Sci Med 1991;33:909-23. [DOI] [PubMed] [Google Scholar]