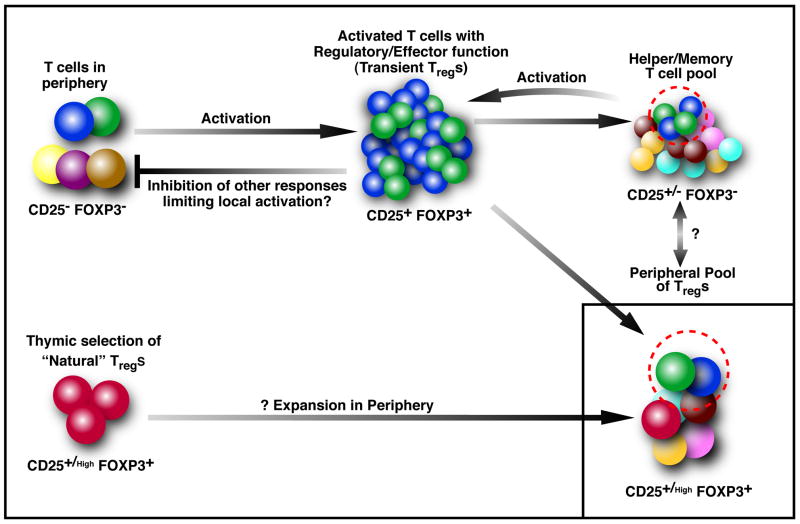
Figure 1. A model of peripheral Treg formation in adult humans, as a consequence of activation.
Every time there is activation of T cells in the periphery, the responding T cells upregulate FOXP3 and CD25 transiently. This is associated with transient suppressive activity and also some effector functions. The suppressive activity may serve to inhibit untoward responses from lower affinity/potentially cross-reactive T cells. During the response, only a subset of these activated T cells retain FOXP3 and contribute to the peripheral pool of FOXP3+ Tregs, persisting for longer periods in the FOXP3+ suppressive state. The majority of the activated cells downregulate FOXP3, contract in numbers and contribute to the peripheral memory pool. Such memory cells have the potential to contribute to the peripheral Treg pool in future activation cycles. Of note, the T cell receptor distribution of the memory pool and the Treg pool are similar, suggesting common precursors. It is still unclear whether there is direct conversion of “stable” Tregs into memory and/or effector cells in vivo. In the thymus, positive selection of T cells with intermediate affinity for self antigens results in the formation of a population of natural FOXP3+ Tregs. While these may be a major source of peripheral Tregs in mice and probably in human infants, most of these may not persist during adult life in humans. Thus, peripherally generated Tregs with diverse antigenic specificities are the major source of circulating Tregs in adult human beings.