Abstract
Rheumatoid arthritis is a chronic inflammatory disorder whose origin of defect has been the subject of extensive research during the past few decades. While a number of immune and non‐immune cell types participate in the development of chronic destructive inflammation in the arthritic joint, synovial fibroblasts have emerged as key effector cells capable of modulating both joint destruction and propagation of inflammation. Ample evidence of aberrant changes in the morphology and biochemical behaviour of rheumatoid arthritis synovial fibroblasts have established the tissue evading and “transformed” character of this cell type. We have recently demonstrated that actin cytoskeletal rearrangements determine the pathogenic activation of synovial fibroblasts in modelled TNF‐mediated arthritis, a finding correlating with similar gene expression changes which we observed in human rheumatoid arthritis synovial fibroblasts. Here, we show that pharmacological inhibition of actin cytoskeleton dynamics alters potential pathogenic properties of the arthritogenic synovial fibroblast, such as proliferation, migration and resistance to apoptosis, indicating novel opportunities for therapeutic intervention in arthritis. Recent advances in this field of research are reviewed and discussed.
Rheumatoid arthritis (RA) represents one of the most common chronic inflammatory disorders, affecting approximately 1% of the population worldwide, thus creating a substantial personal, social and economical burden.1 It is a chronic destructive arthropathy that manifests primarily as a painful and prolonged inflammation of the synovial membrane, a thin tissue that lines the diarthrodial joints. Inflammation is accompanied by a gradual increase in the thickness of the synovial lining, the surface layer of the synovial tissue, which is located adjacent to and in direct contact with the intra‐articular cavity of the joint. The thickening of the synovial layer, mediated by the intrinsic hyperproliferation of synovial cells and inflammatory infiltrates, gives rise to a pathogenic mass called pannus, comprised primarily of synovial fibroblasts and macrophages, as well as T cells and B cells, which propels the inflammatory machinery to the destruction of bone and cartilage,2 leading to irreversible destruction of joint structure and function.3
It is widely accepted that RA is a systemic disease, with a variety of combinatory determinants being attributed to its aetiopathogenesis. These include individual genetic susceptibility, environmental stimuli, physical stress and defective immune responses.4 Traditional mechanistic concepts have strongly implicated T cell and B cell‐dependent pathways driving development of the disease. Following the success of anticytokine rather than antilymphocyte‐targeted therapies in human RA,5 a role for chronic innate immune perturbations has also gained momentum, with the macrophages as major contributors of the inflammatory cytokine milieu.6 Dissecting the causative molecular mechanisms underlying RA development, the unchallengeable fact is that tumour necrosis factor (TNF) is indeed capable of orchestrating the pathogenic inflammatory cascade. A key translational experiment demonstrating the pathogenic potential of TNF in arthritis was originally provided by the generation of transgenic mice overexpressing human TNF (hTNF‐Tg) and developing chronic, erosive and symmetric polyarthritis with histological characteristics resembling the pathogenic process in human RA.7 This model was also instrumental in predicting the significant efficacy of anti‐TNF therapy in clinical trials.7 In accordance with the phenotype of this transgenic mouse, another murine model of RA, generated by genetic deletion of the AU‐rich regulatory region of the murine TNF gene (TNFΔARE) further established the pivotal significance of TNF in RA as well as in Crohn's‐like inflammatory bowel disease.8 RA development in the absence of the lymphocytic compartment in the two TNF transgenic models8,9 challenged the indispensable role of adaptive immunity in RA and indicated the important role of non‐immune cells, such as synovial fibroblasts in the development of this pathology.
Despite these advances, what exactly keeps TNF fuelling the inflammatory response is still unclear, while the increasing number of non‐responders to anti‐TNF therapy10 points to additional mechanisms existing in RA. As anticytokine therapies target a specific agent or pathway, research has been shifted towards cellular targets and processes that are central to the deleterious events initiated by inflammatory cytokines, such as TNFα.
The synovial fibroblast: a “transformed” cell with a leading role in arthritis
Chronic persistent inflammation, wound healing and cancer exhibit significant common features: new extracellullar matrix (ECM) deposition and expansion of neighbouring mesenchymal cells such as fibroblasts or myofibroblasts;11 stromal fibroblasts being suggested as potential “inducers” of certain carcinomas;12 experimental evidence in genetically modified mice lacking TGF‐βRII in fibroblasts revealed that deregulated expression of fibroblast products (eg, hepatocyte growth factor) influences epithelial function and leads to tumorigenesis, indicating the importance of the homeostatic responses of the stromal compartment in modulating/maintaining the tissue integrity.13
In chronic inflammatory arthritis, a stromal cell type that has emerged with a dominant role in both joint damage and the propagation of inflammation is the synovial fibroblast (SF).14 SFs or fibroblast‐like type B synoviocytes along with macrophages or macrophage‐like type A synoviocytes, predominate the cellular population of the normal synovium. Although the origin of SFs is still debatable, they represent a heterogeneous population of cells in terms of tissue localisation, physiology (intimal and subintimal) and derivation (non‐epithelial, mesenchymal cells) that display differential activation and differentiation properties.15 While the primary physiological role of SFs is to provide a nourishing environment for the cartilage and proteoglycans that lubricate the articular surfaces, they also anchor to the ECM through the expression of cell adhesion molecules, thus regulating the cellular population that enters the synovial fluid space. In the context of RA, an immune or environmental trigger may lead to the activation of SFs. This in turn results in inappropriate production of chemokines, adhesion molecules and matrix‐degrading components, mainly matrix metalloproteinases (MMPs) and cathepsins, contributing to the subsequent cartilage and joint destruction.16 Indeed, synovial hyperplasia, a hallmark of RA, has been shown to precede the accumulation of inflammatory cells, suggesting a primary role for SFs in RA aetiology.17,18
Notwithstanding the notion that cytokines like TNFα can trigger SF activation and proliferation,19,20 it seems that SFs can maintain their activation status without the need of continuous stimulation from the pro‐inflammatory microenvironment. This capability of SFs was originally documented by studies on the severe combined immunodeficient (SCID) mouse co‐implantation model of RA.21 In that model it was elegantly shown that co‐implantation of isolated human rheumatoid arthritis synovial fibroblasts (RASFs) and normal human cartilage in the knee joint of healthy SCID mice resulted in the induction of an RA‐like phenotype through the production of cartilage‐destroying enzymes from the proliferating RASFs. Consistent with the latter finding, we have observed a similar effect by transferring immortalised SFs from our well‐established T cell/B cell‐independent transgenic animal model,22 to the knee joint of histocompatible normal recipients. Interestingly, the injected SFs were also able to migrate to other parts of the body, such as the ankle joint, and cause the development of an arthritis‐like disease similar to that of the donor.23 The ability of RASFs to migrate, invade and destroy neighbouring articular cartilage resembles to a great extent that of cancer cells and the process of metastasis.24
Despite the tumour‐like behaviour of the activated RASF, there is little evidence so far in favour of its function as a malignant cell.25 Presently, no clear evidence exists regarding gene rearrangements and microsatellite instability,26 which is well reported for cancer cells.27,28 The identification of abnormal gene expression of a number of proto‐oncogenes, such as ras, raf, sis, myb, c‐myc, c‐fos and c‐jun in the RA synovium29,30,31 and the association of RA and somatic mutations in the tumour‐suppressor gene p53,32 do not provide conclusive evidence for its characterisation as a malignant cell. Moreover, mutations in PTEN, a tumour suppressor gene, have been described in several human cancers, and have been associated with the invasiveness and metastatic properties of malignant tumours.33,34 Interestingly, non‐genetically related lack of expression of PTEN at sites of SF invasion further corroborated the argument that defective apoptosis may largely account for the synovial hyperplasia in RA.35 Apart from the hyperproliferation status of RASFs, shown by their fast growth in vitro and the increased expression of factors such as c‐myc and NF‐κB,30,36 resistance to apoptosis provides an alternative scenario for SF hyperplasia. Defective apoptosis was well documented by the site‐specific upregulation of several anti‐apoptotic factors, including Sentrin‐1, Bcl‐2, and Flip, where synovial invasive growth and destruction is evident.37,38,39
Another intriguing characteristic of the “transformed” RASF stems from its ability to produce a variety of pro‐angiogenic factors like FGF, VEGF, TGF‐β, GM‐CSF that are necessary for the generation of blood vessels fuelling the destructive growth of the pannus and RA.40 Consistent with the latter is the previously reported finding of anchorage‐independent growth and loss of contact cell inhibition, suggesting that RASFs acquire characteristics found in transformed cells with a tumorigenic potential.41 Moreover, the role of epithelial–mesenchymal transition (EMT) that has been widely discussed lately in terms of stromal‐associated carcinogenesis,42,43 is presently mentioned as a potential mechanism for the development of several stromal‐related rheumatic diseases, such as RA.44 The upregulation of TGF‐β and activation of its pathway in arthritic synovium,45,46 the stress fibre formation and the myofibroblastoid α smooth muscle actin expression47,48,49,50 as well as the MMP secretion in RASFs, point to the reprogramming occuring during EMT.51 However, the absence of epithelial characteristics in the synovium is puzzling. Consequently, the term “transformed” has most commonly been attributed to the activated RASF in order to summarise its unique characteristics that contribute to RA pathogenesis.24
Functional genomics points towards the actin cytoskeleton machinery
The distinct alteration in the cellular physiology of RASFs can also be viewed in the context of their interaction with the ECM. The latter, coupled with components of the cytoskeleton, provides the necessary hydraulic resistance to the mechanical pressure opposed to the growing pannus and the synovial layer, thereby holding the synovial fluid within the joint cavity while it may trap inflammatory molecules.52 Furthermore, loss of ECM–SF adhesion has been shown to affect focal adhesion kinases, causing a potential resistance to apoptosis, otherwise conferred by these factors.53 Indeed, the capability of cells like the SFs to adhere to the ECM reflects their status of cytoskeleton organisation and cellular morphology,54 while it may also be related to changes in their migration and proliferation potential.55 Interestingly, our recent high‐throughput expression profiling analysis on SFs derived from the hTNF‐Tg animal model, revealed a number of deregulated genes, including gsn, aqp1, cdc42hom, eef1a1, tuba1, rab14, lsp1, lst1, mylc2b, pitpnm and pstpip1, which are known to be involved in actin filament and cytoskeleton organisation.56 More importantly, the expression status of a vast majority of these genes is being further replicated and validated in an independent cohort of patients with RA (unpublished data), hinting that there is a simultaneous alteration in RASFs' specific gene expression status and actin cytoskeleton rearrangements.
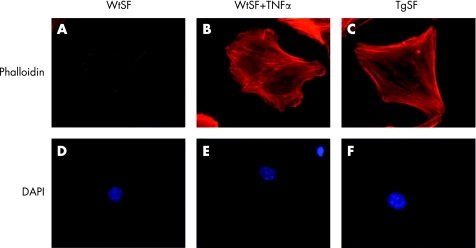
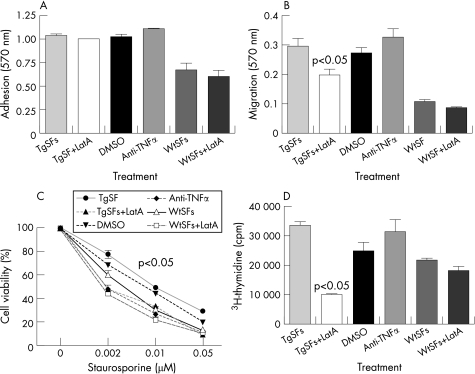
Notably visualisation of F‐actin in vitro on both wild type (Wt) and hTNF‐Tg SFs (TgSFs), shows a substantial increase in stress fibre formation in TgSFs compared to WtSFs, indicating altered actin dynamics in TgSFs (fig 1A, C).56 A similar outcome is deduced by the treatment of WtSFs with recombinant human TNFα (fig 1B), suggesting that the “transformed” morphology of the SFs can be triggered by TNFα. This complements the previously reported finding that recombinant TNFα can induce morphological changes and increased proliferation of SFs.19 Most importantly, we have previously shown that TgSFs have an aberrant phenotype, compared with those of Wt mice. We have observed TgSFs to be more adhesive and to show greater motility and proliferation potential compared to Wt.56 In vitro analysis on the effect of latrunculin A (Lat‐A), an inhibitor of F‐actin polymerisation,57 has indicated the potential of actin‐modulating drugs in altering the cytoskeletal properties of TgSFs. More specifically, treatment with Lat‐A results in decreased migration of TgSFs, while adhesion seems to be unaffected compared to WtSFs (fig 2A, B). This is in keeping with previous findings of Lat‐A treatment and myofibrils.58 At the same time, TgSFs treated with anti‐TNFα do not show altered migration properties, suggesting that this effect might not be TNFα‐driven. Alternatively, this could indicate that there is an imprinted phenotype in the TgSF cells due to the chronic overexpression of TNFα, which cannot be reversed by a short treatment with anti‐TNFα antibody. In terms of other properties, treatment with Lat‐A seems to have a dramatic effect on the proliferation levels of TgSFs which decline even lower than the levels of WtSFs, while DMSO and TNFα do not have any effect on the rate of proliferation of TgSFs (fig 2D). Most importantly, apoptosis assays using staurosporine as an inducer of cell death revealed that Lat‐A disruption of the TgSFs cytoskeleton causes decreased resistance to apoptosis at the levels of the WtSFs (fig 2C). This result is consistent with previous findings showing that treatment with cytochalasin D (another inhibitor of actin polymerisation) enhances apoptosis in HeLa cells, while phalloidin (an inducer of actin polymerisation) does not affect apoptosis.59 Notably, defective migration potential and abnormal cytoskeleton organisation are unique characteristics that define the “transformed” state of a cell.60 Since an actin cytoskeleton‐disrupting drug, like Lat‐A, can cause profound changes in the properties of the “transformed” SF and act independently of TNFα, it is only rational to suggest that treating patients with RA with a combination of anti‐TNF and a drug that targets the actin cytoskeleton may be more effective than anti‐TNFα treatment alone.
Figure 1 Immunofluorescence of stress fibre formation on wild type, wild type treated with hTNFα and hTNF‐Tg primary SFs, respectively. (Details on primary SF isolation, F‐actin and DAPI staining procedures have been shown previously.)56
Figure 2 Application of latrunculin A on the “transformed” synovial fibroblast. Disruption of actin cytoskeleton was induced with 4 µg/ml latrunculin A (Sigma) for 2 h at 37°C as previously described.92 As control of TNFα involvement in the actin cytoskeleton organisation 1 μg/ml anti‐TNF (infliximab) was added on TgSFs for the same period of time. (A, B, D) Adhesion, migration and proliferation assays were performed as previously described.56 (C) Apoptosis assays were performed by treating cells with 0.002–0.05 μM staurosporine, for 24 h at 37°C overnight. Cell survival was determined using the crystal violet assay as before.8 All results are expressed as the means±SE of one representative experiment performed in triplicate. Statistical analysis was performed using Student t test, with a level of significance set at p<0.05.
The actin cytoskeleton network in the context of RA
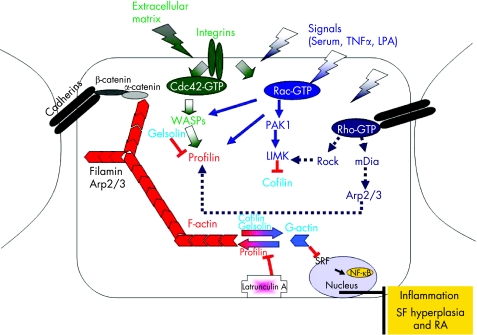
As already discussed, the actin cytoskeleton plays an essential role in the homeostasis and normal function of the cell. Processes such as cell shape modulation, differentiation, migration, development, wound healing and maintenance of polarity are greatly dependent on the actin cytoskeleton. To perform these functions properly, the cell has to be able to respond efficiently to all extracellular and intracellular stimuli and modulate its actin cytoskeleton accordingly. For instance, it has to be able to modulate the amount of filamentous F‐actin versus monomeric G‐actin and the number of stress fibres in order to be able to move. For that purpose, a complex intracellular protein network has been established, enabling the cell to modulate actin polymerisation, depolymerisation, nucleation as well as actin organisation depending on the cell's needs. Since these processes are crucial for the cell homeostasis and survival, it is not surprising that more than one protein regulates each step. For example, profilin, thymosin β,61 α‐actinin and VASP62,63,64 mediate actin polymerisation,61 while cofilin/ADF,61 gelsolin,61 and caspase 1165 are critical for actin depolymerisation, and filamin,66 and Arp2/361 modulate the nucleation of actin bundles (fig 3). Apart from the control of actin polymerisation, modulation of actin organisation is also of utmost importance. Thus, the actin cytoskeleton structure can be modified by a number of signalling and/or actin‐binding proteins. This results in stress fibre, focal adhesion, lamellipodia or filopodia formation depending on the specific requirements of the cell at the specific time. The assembly of projections in the membrane surrounding the cell is regulated mainly by small GTPases of the Rho family of proteins, Rac1, Rho and Cdc42.67,68,69 The Rho family of proteins belongs to the Ras protein superfamily and its members are found in different isoforms. Each of these proteins has the capacity to bind to GTP and constantly be converted from an active GTP‐bound form to an inactive GDP‐bound form. In the active state they can interact with a number of effector molecules in order to transmit a specific signal and have a specific outcome. For instance, two members of this family, Rac1 and Cdc42, when activated by growth factors or by integrins, the receptors that connect the ECM with the inner compartment of the cell, mediate the lamellipodia and filopodia formation, respectively, while Rho is responsible for the stress fibre formation.67,70
Figure 3 A schematic representation of the proposed interaction between actin cytoskeleton rearrangements and the maintenance of rheumatoid arthritis pathogenesis.
In the context of RA, it was recently shown that in human cultured RASFs, TNFα‐induced activation of NF‐κB and cytokine secretion is dependent on the activation of RhoA, suggesting a central role of this GTPase in the arthritic inflammatory response.71 As RhoA induces stress fibre formation, it is remarkable that the p65/RelA subunit of NF‐κB co‐localises and binds to F‐actin, while re‐establishment of the actin cytoskeleton organisation seems to bring back to normal the distribution of p65 in the cytoplasm.72 Moreover, our studies have shown that depletion of gelsolin, an actin‐binding protein known to be involved in actin depolymerisation by inhibiting F‐actin formation, from mice with RA resulted in exacerbation of the disease.56 This suggests a critical role for gelsolin in modulating the actin cytoskeleton in RASFs and thus in the progression of RA. The importance of tissue remodelling in RA was further reinforced by recent experiments on cadherin‐11‐deficient mice, which showed diminished adhesion, migration and invasion properties of RASFs, while they seem to confer resistance to K/B×N serum transfer model of arthritis.73 Interestingly, the effect of the actin cytoskeleton on both cell morphology and gene transcription has been the subject of an elegant study, involving Mal, a regulator of gene expression. It was shown that upon F‐actin formation, Mal dissociates from G‐actin and activates the serum response factor (SRF), whose target genes range from genes regulating cell growth, proliferation and differentiation to genes that regulate the actin cytoskeleton machinery.74 Not surprisingly, ablation of the SRF in murine embryonic stem cells resulted in low F‐actin levels and reduced adhesion and migration capabilities, thus documenting a dynamic link between SRF and remodelling of the actin cytoskeleton.75
In light of the evidence discussed so far, we propose a model whereby an immune or environmental stimulus could trigger TNFα to induce the reorganisation of the actin cytoskeleton, through activation of small GTPases, which may lead to morphological changes in the SFs, ultimately resulting in the “transformed” phenotype of SFs characterised by increased migration, adhesion and proliferation properties (fig 3). Furthermore, increased F‐actin formation could lead to increased gene expression mediated by SRF and NF‐κB, resulting in sustaining the disorganised actin cytoskeleton and then in the production of pro‐inflammatory cytokines, thereby maintaining the detrimental effects of the arthritogenic response (fig 3).
The essential role of the actin cytoskeleton in maintaining cell homeostasis and survival, as well as the role of the multitude of proteins that are crucial for fine‐tuning its organisation, is also manifested very clearly in several disease states, other than RA. A recessively inherited genetic disorder known as neutrophil actin dysfunction has been linked to a severe defect in actin assembly and polymerisation in the polymorphonuclear leucocytes, showing the significance of actin polymerisation for neutrophil motility.76 In line with neutrophil actin dysfunction, it has been established that a missense point mutation in the gene encoding β‐actin that alters depolymerisation dynamics is associated with an autosomal dominant disease characterised by developmental malformations, deafness and dystonia syndrome, where co‐contraction of agonistic and antagonistic muscles occurs.77 Furthermore, in recent studies in which the neuropathology of brains from identical twins with dystonia was examined, cofilin and actin aggregates were found within the neocortex.78 Notably, some of these structures showed typical rod‐like morphology and sizes similar to those found in the brains of patients with Alzheimer's disease.79 Also of interest is the fact that the dominantly inherited familial amyloidosis Finnish type is attributed to the accumulation of a 71‐amino acid amyloidogenic fragment of mutant gelsolin.80 Interestingly, serum amyloid A was found to be elevated in patients with RA,81 with recent evidence suggesting an induction of synovial hyperplasia and angiogenesis as an outcome of its elevation.82
Not surprisingly, actin and/or actin‐binding protein‐related disorders have been associated with cancer progression and metastasis. Just like in RA, many of the changes occurring during cancer progression as well as during the metastatic process are linked to deregulations in actin cytoskeleton and the actin‐binding proteins at cell–matrix or cell–cell adhesion sites.83,84 Reduction in the expression level of the cell–cell adhesion molecule E‐cadherin, for example, is known to be associated with cancer progression and poor prognosis.85,86 Moreover, deregulation in several ECM adhesion proteins that directly or indirectly associate with actin have been shown to be critical for cancer progression and metastasis, either by affecting cancer cell proliferation and apoptosis rates or by inducing anoikis, a type of apoptosis induced by loss of contact and adhesion to the ECM.87,88,89,90 Some of them have been proposed as potential targets for future therapeutical anti‐cancer interventions.91
Conclusions
Accumulating evidence suggests that there is a direct but complex interaction between the actin cytoskeleton and the arthritic SF, of a yet unknown nature. Gaining a better understanding of the role of the actin cytoskeleton in modulating the SF function to initiate and maintain the arthritogenic response may provide new insights into novel therapeutic modalities. A rational strategy to achieve that goal would be the inhibition of signalling players that induce actin polymerisation by small peptide molecules or by interfering directly with their gene expression. Functional and in vivo studies will hopefully uncover such targets, whose impact on RA therapy remains to be elucidated.
Abbreviations
ECM - extracellular matrix
EMT - epithelial–mesenchymal transition
Lat‐A - latrunculin A
MMPs - matrix metalloproteinases
RA - rheumatoid arthritis
RASFs - rheumatoid arthritis synovial fibroblasts
SF - synovial fibroblast
SCID - severe combined immunodeficient
SRF - serum response factor
TNFα - tumour necrosis factor α
Wt - wild type
Footnotes
Competing interests: None declared.
References
- 1.Silman A J, Pearson J E. Epidemiology and genetics of rheumatoid arthritis. Arthritis Res 20024(Suppl 3)S265–S272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tak P P. Examination of the synovium and synovial fluid. In: Firestein GS, Panayi GS, Wollheim FA, eds. Rheumatoid arthritis. Frontiers in pathogenesis and treatment. New York: Oxford University Press, 200055–68.
- 3.Firestein G S. Rheumatoid synovitis and pannus. In: Klippel JH, Dieppe PA, eds. Rheumatology. London: Mosby, 19985.13.1–5.1324.
- 4.Firestein G S. Evolving concepts of rheumatoid arthritis. Nature 2003423356–361. [DOI] [PubMed] [Google Scholar]
- 5.Strand V, Kimberly R, Isaacs J D. Biologic therapies in rheumatology: lessons learned, future directions. Nat Rev Drug Discov 2007675–92. [DOI] [PubMed] [Google Scholar]
- 6.Feldmann M, Brennan F M, Maini R N. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol 199614397–440. [DOI] [PubMed] [Google Scholar]
- 7.Elliott M J, Maini R N, Feldmann M, Long‐Fox A, Charles P, Katsikis P.et al Treatment of rheumatoid arthritis with chimeric monoclonal antibodies to tumor necrosis factor alpha. Arthritis Rheum 1993361681–1690. [DOI] [PubMed] [Google Scholar]
- 8.Kontoyiannis D, Pasparakis M, Pizarro T T, Cominelli F, Kollias G. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU‐rich elements: implications for joint and gut‐associated immunopathologies. Immunity 199910387–398. [DOI] [PubMed] [Google Scholar]
- 9.Kollias G, Douni E, Kassiotis G, Kontoyiannis D. On the role of tumor necrosis factor and receptors in models of multiorgan failure, rheumatoid arthritis, multiple sclerosis and inflammatory bowel disease. Immunol Rev 1999169175–194. [DOI] [PubMed] [Google Scholar]
- 10.Lipsky P E, van der Heijde D M, St Clair E W, Furst D E, Breedveld F C, Kalden J R.et al Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti‐Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med 20003431594–1602. [DOI] [PubMed] [Google Scholar]
- 11.Dvorak H F. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 19863151650–1659. [DOI] [PubMed] [Google Scholar]
- 12.Bhowmick N A, Neilson E G, Moses H L. Stromal fibroblasts in cancer initiation and progression. Nature 2004432332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhowmick N A, Chytil A, Plieth D, Gorska A E, Dumont N, Shappell S.et al TGF‐beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science 2004303848–851. [DOI] [PubMed] [Google Scholar]
- 14.Kontoyiannis D, Kollias G. Fibroblast biology: synovial fibroblasts in rheumatoid arthritis – leading role or chorus line? Arthritis Res 20002342–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang H Y, Chi J T, Dudoit S, Bondre C, van de Rijn M, Botstein D.et al Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc Natl Acad Sci USA 20029912877–12882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ritchlin C. Fibroblast biology: effector signals released by the synovial fibroblast in arthritis. Arthritis Res 20002356–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopez‐Bote J P, Bernabeu C, Marquet A, Fernandez J M, Larraga V. Adjuvant‐induced polyarthritis. Synovial cell activation prior to polyarthritis onset. Arthritis Rheum 198831769–775. [DOI] [PubMed] [Google Scholar]
- 18.Marinova‐Mutafchieva L, Williams R O, Funa K, Maini R N, Zvaifler N J. Inflammation is preceded by tumor necrosis factor‐dependent infiltration of mesenchymal cells in experimental arthritis. Arthritis Rheum 200246507–513. [DOI] [PubMed] [Google Scholar]
- 19.Butler D M, Piccoli D S, Hart P H, Hamilton J A. Stimulation of human synovial fibroblast DNA synthesis by recombinant human cytokines. J Rheumatol 1988151463–1470. [PubMed] [Google Scholar]
- 20.Dayer J M, Krane S M, Russell R G, Robinson D R. Production of collagenase and prostaglandins by isolated adherent rheumatoid synovial cells. Proc Natl Acad Sci USA 197673945–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muller‐Ladner U, Kriegsmann J, Franklin B N, Matsumoto S, Geiler T, Gay R E.et al Synovial fibroblasts of patients with rheumatoid arthritis attach to and invade normal human cartilage when engrafted into SCID mice. Am J Pathol 19961491607–1615. [PMC free article] [PubMed] [Google Scholar]
- 22.Keffer J, Probert L, Cazlaris H, Georgopoulos S, Kaslaris E, Kioussis D.et al Transgenic mice expressing human tumour necrosis factor: a predictive genetic model of arthritis. Embo J 1991104025–4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aidinis V, Plows D, Haralambous S, Armaka M, Papadopoulos P, Kanaki M Z.et al Functional analysis of an arthritogenic synovial fibroblast. Arthritis Res 20035R140–R157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Firestein G S. Invasive fibroblast‐like synoviocytes in rheumatoid arthritis. Passive responders or transformed aggressors? Arthritis Rheum 1996391781–1790. [DOI] [PubMed] [Google Scholar]
- 25.Taniguchi K, Kohsaka H, Inoue N, Terada Y, Ito H, Hirokawa K.et al Induction of the p16INK4a senescence gene as a new therapeutic strategy for the treatment of rheumatoid arthritis. Nat Med 19995760–767. [DOI] [PubMed] [Google Scholar]
- 26.Kullmann F, Widmann T, Kirner A, Justen H P, Wessinghage D, Dietmaier W.et al Microsatellite analysis in rheumatoid arthritis synovial fibroblasts. Ann Rheum Dis 200059386–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woerner S M, Kloor M, von Knebel Doeberitz M, Gebert J F. Microsatellite instability in the development of DNA mismatch repair deficient tumors. Cancer Biomark 2006269–86. [DOI] [PubMed] [Google Scholar]
- 28.Aplan P D. Causes of oncogenic chromosomal translocation. Trends Genet 20062246–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muller‐Ladner U, Gay R E, Gay S. Activation of synoviocytes. Curr Opin Rheumatol 200012186–194. [DOI] [PubMed] [Google Scholar]
- 30.Qu Z, Garcia C H, O'Rourke L M, Planck S R, Kohli M, Rosenbaum J T. Local proliferation of fibroblast‐like synoviocytes contributes to synovial hyperplasia. Results of proliferating cell nuclear antigen/cyclin, c‐myc, and nucleolar organizer region staining. Arthritis Rheum 199437212–220. [DOI] [PubMed] [Google Scholar]
- 31.Xue C, Takahashi M, Hasunuma T, Aono H, Yamamoto K, Yoshino S.et al Characterisation of fibroblast‐like cells in pannus lesions of patients with rheumatoid arthritis sharing properties of fibroblasts and chondrocytes. Ann Rheum Dis 199756262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamanishi Y, Boyle D L, Rosengren S, Green D R, Zvaifler N J, Firestein G S. Regional analysis of p53 mutations in rheumatoid arthritis synovium. Proc Natl Acad Sci USA 20029910025–10030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang S I.et al PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 19972751943–1947. [DOI] [PubMed] [Google Scholar]
- 34.Steck P A, Pershouse M A, Jasser S A, Yung W K, Lin H, Ligon A H.et al Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet 199715356–362. [DOI] [PubMed] [Google Scholar]
- 35.Pap T, Franz J K, Hummel K M, Jeisy E, Gay R, Gay S. Activation of synovial fibroblasts in rheumatoid arthritis: lack of expression of the tumour suppressor PTEN at sites of invasive growth and destruction. Arthritis Res 2000259–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muller‐Ladner U, Gay R E, Gay S. Role of nuclear factor kappaB in synovial inflammation. Curr Rheumatol Rep 20024201–207. [DOI] [PubMed] [Google Scholar]
- 37.Franz J K, Pap T, Hummel K M, Nawrath M, Aicher W K, Shigeyama Y.et al Expression of sentrin, a novel antiapoptotic molecule, at sites of synovial invasion in rheumatoid arthritis. Arthritis Rheum 200043599–607. [DOI] [PubMed] [Google Scholar]
- 38.Pope R M. Apoptosis as a therapeutic tool in rheumatoid arthritis. Nat Rev Immunol 20022527–535. [DOI] [PubMed] [Google Scholar]
- 39.Catrina A I, Ulfgren A K, Lindblad S, Grondal L, Klareskog L. Low levels of apoptosis and high FLIP expression in early rheumatoid arthritis synovium. Ann Rheum Dis 200261934–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koch A E. Angiogenesis as a target in rheumatoid arthritis. Ann Rheum Dis 200362(Suppl 2)ii60–ii67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lafyatis R, Remmers E F, Roberts A B, Yocum D E, Sporn M B, Wilder R L. Anchorage‐independent growth of synoviocytes from arthritic and normal joints. Stimulation by exogenous platelet‐derived growth factor and inhibition by transforming growth factor‐beta and retinoids. J Clin Invest 1989831267–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kang Y, Massague J. Epithelial‐mesenchymal transitions: twist in development and metastasis. Cell 2004118277–279. [DOI] [PubMed] [Google Scholar]
- 43.Thiery J P. Epithelial‐mesenchymal transitions in tumour progression. Nat Rev Cancer 20022442–454. [DOI] [PubMed] [Google Scholar]
- 44.Zvaifler N J. Relevance of the stroma and epithelial‐mesenchymal transition (EMT) for the rheumatic diseases. Arthritis Res Ther 20068210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taketazu F, Kato M, Gobl A, Ichijo H, ten Dijke P, Itoh J.et al Enhanced expression of transforming growth factor‐beta s and transforming growth factor‐beta type II receptor in the synovial tissues of patients with rheumatoid arthritis. Lab Invest 199470620–630. [PubMed] [Google Scholar]
- 46.Pohlers D, Beyer A, Koczan D, Wilhelm T, Thiesen H J, Kinne R W. Constitutive upregulation of the TGF‐b pathway in rheumatoid arthritis synovial fibroblasts. Arthritis Res Ther 20079R59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kasperkovitz P V, Timmer T C, Smeets T J, Verbeet N L, Tak P P, van Baarsen L G.et al Fibroblast‐like synoviocytes derived from patients with rheumatoid arthritis show the imprint of synovial tissue heterogeneity: evidence of a link between an increased myofibroblast‐like phenotype and high‐inflammation synovitis. Arthritis Rheum 200552430–441. [DOI] [PubMed] [Google Scholar]
- 48.Mattey D L, Dawes P T, Nixon N B, Slater H. Transforming growth factor beta 1 and interleukin 4 induced alpha smooth muscle actin expression and myofibroblast‐like differentiation in human synovial fibroblasts in vitro: modulation by basic fibroblast growth factor. Ann Rheum Dis 199756426–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bauer S, Jendro M C, Wadle A, Kleber S, Stenner F, Dinser R.et al Fibroblast activation protein is expressed by rheumatoid myofibroblast‐like synoviocytes. Arthritis Res Ther 20068R171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steenvoorden M M, Tolboom T C, van der Pluijm G, Lowik C, Visser C P, DeGroot J.et al Transition of healthy to diseased synovial tissue in rheumatoid arthritis is associated with gain of mesenchymal/fibrotic characteristics. Arthritis Res Ther 20068R165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Radisky D C, Levy D D, Littlepage L E, Liu H, Nelson C M, Fata J E.et al Rac1b and reactive oxygen species mediate MMP‐3‐induced EMT and genomic instability. Nature 2005436123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Konttinen Y T, Li T ‐ F, Hukkanen M, Ma J, Xu J ‐ W, Virtanen I. Fibroblast biology: signals targeting the synovial fibroblast in arthritis. Arthritis Res 20002348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Frisch S M, Vuori K, Ruoslahti E, Chan‐Hui P Y. Control of adhesion‐dependent cell survival by focal adhesion kinase. J Cell Biol 1996134793–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gumbiner B M. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell 199684345–357. [DOI] [PubMed] [Google Scholar]
- 55.Lelievre S A, Bissell M J, Pujuguet P. Cell nucleus in context. Crit Rev Eukaryot Gene Expr 20001013–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aidinis V, Carninci P, Armaka M, Witke W, Harokopos V, Pavelka N.et al Cytoskeletal rearrangements in synovial fibroblasts as a novel pathophysiological determinant of modeled rheumatoid arthritis. PLoS Genet 20051e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Giganti A, Friederich E. The actin cytoskeleton as a therapeutic target: state of the art and future directions. Prog Cell Cycle Res 20035511–525. [PubMed] [Google Scholar]
- 58.Wang J, Sanger J M, Sanger J W. Differential effects of Latrunculin‐A on myofibrils in cultures of skeletal muscle cells: insights into mechanisms of myofibrillogenesis. Cell Motil Cytoskeleton 20056235–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kulms D, Dussmann H, Poppelmann B, Stander S, Schwarz A, Schwarz T. Apoptosis induced by disruption of the actin cytoskeleton is mediated via activation of CD95 (Fas/APO‐1). Cell Death Differ 20029598–608. [DOI] [PubMed] [Google Scholar]
- 60.Puck T T, Krystosek A. Role of the cytoskeleton in genome regulation and cancer. Int Rev Cytol 199213275–108. [DOI] [PubMed] [Google Scholar]
- 61.Disanza A, Steffenc A, Hertzoga M, Frittoli E, Rottnerd K, Scitaa G. Actin polymerization machinery: the finish line of signaling networks, the starting point of cellular movement. Cell Mol Life Sci 200562955–970. [DOI] [PubMed] [Google Scholar]
- 62.Castellano F, Le Clainche C, Patin D, Carlier M, Chavrier P. A WASP–VASP complex regulates actin polymerization at the plasma membrane. Embo J 2001205603–5614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Krause M, Bear J, Loureiro J, Gertler F. The Ena/VASP enigma. J Cell Sci 20021154721–4726. [DOI] [PubMed] [Google Scholar]
- 64.Kwiatkowski A V, Gertler F B, Loureiro J J. Function and regulation of Ena/VASP proteins. Trends Cell Biol 200313386–392. [DOI] [PubMed] [Google Scholar]
- 65.Li J, Brieher W M, Scimone M L, Kang S J, Zhu H, Yin H.et al Caspase‐11 regulates cell migration by promoting Aip1–Cofilin‐mediated actin depolymerization. Nat Cell Biol 20079276–286. [DOI] [PubMed] [Google Scholar]
- 66.Stossel T P, Condeelis J, Cooley L, Hartwig J H, Noegel A, Schleicher M.et al Filamins as integrators of cell mechanics and signalling. Nat Rev Mol Cell Biol 20012138–145. [DOI] [PubMed] [Google Scholar]
- 67.Bishop A, Hall A. Rho GTPases and their effector proteins. Biochem J 2000348241–255. [PMC free article] [PubMed] [Google Scholar]
- 68.Hall A. Rho GTPases and the actin cytoskeleton. Science 1998279509–514. [DOI] [PubMed] [Google Scholar]
- 69.Ridley A J. Rho family proteins: coordinating cell responses. Trends Cell Biol 200111471–477. [DOI] [PubMed] [Google Scholar]
- 70.Small J V, Stradal T, Vignal E, Rottner K. The lamellipodium: where motility begins. Trends Cell Biol 200212112–120. [DOI] [PubMed] [Google Scholar]
- 71.Xu H, Liu P, Liang L, Danesh F R, Yang X, Ye Y.et al RhoA‐mediated, tumor necrosis factor alpha‐induced activation of NF‐kappaB in rheumatoid synoviocytes: inhibitory effect of simvastatin. Arthritis Rheum 2006543441–3451. [DOI] [PubMed] [Google Scholar]
- 72.Are A F, Galkin V E, Pospelova T V, Pinaev G P. The p65/RelA subunit of NF‐kappaB interacts with actin‐containing structures. Exp Cell Res 2000256533–544. [DOI] [PubMed] [Google Scholar]
- 73.Lee D M, Kiener H P, Agarwal S K, Noss E H, Watts G F, Chisaka O.et al Cadherin‐11 in synovial lining formation and pathology in arthritis. Science 20073151006–1010. [DOI] [PubMed] [Google Scholar]
- 74.Vartiainen M K, Guettler S, Larijani B, Treisman R. Nuclear actin regulates dynamic subcellular localization and activity of the SRF cofactor MAL. Science 20073161749–1752. [DOI] [PubMed] [Google Scholar]
- 75.Schratt G, Philippar U, Berger J, Schwarz H, Heidenreich O, Nordheim A. Serum response factor is crucial for actin cytoskeletal organization and focal adhesion assembly in embryonic stem cells. J Cell Biol 2002156737–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Southwick F S, Dabiri G A, Stossel T P. Neutrophil actin dysfunction is a genetic disorder associated with partial impairment of neutrophil actin assembly in three family members. J Clin Invest 1988821525–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Procaccio V, Salazar G, Ono S, Styers M L, Gearing M, Davila A.et al A mutation of β‐actin that alters depolymerization dynamics is associated with autosomal dominant developmental malformations, deafness, and dystonia. Am J Hum Genet 200678947–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gearing M, Juncos J L, Procaccio V, Gutekunst C A, Marino‐Rodriguez E M, Gyure K A.et al Aggregation of actin and cofilin in identical twins with juvenile‐onset dystonia. Ann Neurol 200252465–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bamburg J R, Wiggan O P. ADF/cofilin and actin dynamics in disease. Trends Cell Biol 200212598–605. [DOI] [PubMed] [Google Scholar]
- 80.de la Chapelle A, Tolvanen R, Boysen G, Santavy J, Bleeker‐Wagemakers L, Maury C P J.et al Gelsolin‐derived familial amyloidosis caused by asparagine or tyrosine substitution for aspartic acid at residue 187. Nat Genet 19922157–160. [DOI] [PubMed] [Google Scholar]
- 81.Vallon R, Freuler F, Desta‐Tsedu N, Robeva A, Dawson J, Wenner P.et al Serum amyloid A (apoSAA) expression is up‐regulated in rheumatoid arthritis and induces transcription of matrix metalloproteinases. J Immunol 20011662801–2807. [DOI] [PubMed] [Google Scholar]
- 82.Lee M S, Yoo S A, Cho C S, Suh P G, Kim W U, Ryu S H. Serum amyloid A binding to formyl peptide receptor‐like 1 induces synovial hyperplasia and angiogenesis. J Immunol 20061775585–5594. [DOI] [PubMed] [Google Scholar]
- 83.Akiyama T, Kawasaki Y. Wnt signalling and the actin cytoskeleton. Oncogene 2006257538–7544. [DOI] [PubMed] [Google Scholar]
- 84.Vasiliev J. Cytoskeletal mechanisms responsible for invasive migration of neoplastic cells. Int J Dev Biol 200448425–439. [DOI] [PubMed] [Google Scholar]
- 85.Avizienyte E, Wyke A, Jones R, McLean G, Westho V M, Brunton V.et al Src‐induced de‐regulation of E‐cadherin in colon cancer cells requires integrin signaling. Nat Cell Biol 20024632–638. [DOI] [PubMed] [Google Scholar]
- 86.Bryant D, Stow J L. The ins and outs of E‐cadherin trafficking. Trends Cell Biol 200414427–434. [DOI] [PubMed] [Google Scholar]
- 87.Attwell S, Roskelley C, Dedhar S. The integrin‐linked kinase (ILK) suppresses anoikis. Oncogene 2000193811–3815. [DOI] [PubMed] [Google Scholar]
- 88.Dai D L, Makretsov N, Campos E I, Huang C, Zhou Y, Huntsman D.et al Increased expression of integrin‐linked kinase is correlated with melanoma progression and poor patient survival. Clin Cancer Res 200394409–4414. [PubMed] [Google Scholar]
- 89.Hannigan G, Troussard A A, Dedhar S. Integrin‐linked kinase: a cancer therapeutic target unique among its ILK. Nat Rev Cancer 2005551–63. [DOI] [PubMed] [Google Scholar]
- 90.Kato K, Shiozawa T, Mitsushita J, Toda A, Horiuchi A, Nikaido T.et al Expression of the Mitogen‐Inducible Gene‐2 (mig‐2) is elevated in human uterine leiomyomas but not in leiomyosarcomas. Hum Pathol 20043555–60. [DOI] [PubMed] [Google Scholar]
- 91.Yoganathan T N, Costello P, Chen X, Jabali M, Yan J, Leung D.et al Integrin‐linked kinase (ILK): a “hot” therapeutic target. Biochem Pharmacol 2000601115–1119. [DOI] [PubMed] [Google Scholar]
- 92.Spector I, Shochet N R, Blasberger D, Kashman Y. Latrunculins—novel marine macrolides that disrupt microfilament organization and affect cell growth: I. Comparison with cytochalasin D. Cell Motil Cytoskeleton 198913127–144. [DOI] [PubMed] [Google Scholar]