Abstract
Aims
The objective of this study was to investigate whether baseline receptor activator for nuclear factor kappaB ligand (RANKL) and osteoprotegerin (OPG) serum (s) levels can predict the therapeutic response to TNF antagonists (a‐TNF).
Methods
We studied 75 rheumatoid arthritis patients (81% female) with a longstanding refractory disease. The variables of disease activity, physical function and sRANKL and sOPG levels were determined before and after both 12–14 and 28–30 weeks of a‐TNF therapy (65 adalimumab, 10 infliximab). Remission was defined by a 28 joint count disease activity score (DAS28) ⩽2.6 and clinical response by a reduction in DAS28⩾1.2 at both 3‐ and 7‐month follow‐up visits.
Results
In most patients, disease activity was severe, as reflected by a baseline DAS28 score of 5.9±1 (mean±SD), an HAQ of 1.6 (1.1 to 2.1) (median (interquartile range (IQR))) and a CRP 15 mg/l (IQR: 9 to 24). The sRANKL levels and RANKL/OPG ratio in patients that achieved remission were significantly lower at baseline than in the remaining patients at both 3 and 7 months of follow‐up. The sOPG levels correlated with the HAQ and the physician's disease assessment and diminished significantly after a‐TNF treatment. However, no significant association was detected between the therapeutic response profile and sOPG levels.
Conclusions
These data suggest that in patients receiving a‐TNF treatment, lower serum levels of RANKL and RANKL/OPG ratio may serve to predict remission.
In rheumatoid arthritis (RA), bone destruction in the erosions is mainly due to the abnormal activation of osteoclasts. It is currently known that the control of osteoclast formation and activation is principally mediated by three components of the TNF signalling pathway: the receptor activator for nuclear factor kappaB (RANK), a membrane‐bound osteclast receptor that initiates osteclastic bone resorption after binding its ligand (RANKL) and osteoprotegerin (OPG), the key inhibitor of bone resorption that acts as a soluble, non‐signalling decoy receptor for RANKL.1 Therefore, the balance between OPG and RANKL is a fundamental factor in controlling bone resorption and can be influenced by several hormones and cytokines, including TNF.
Soluble OPG and RANKL can be measured in human serum, and higher levels of these mediators have been described in serum samples from RA patients when compared with healthy individuals.2 Moreover, higher levels of RANKL are also expressed in tissues from patients with active RA and spondyloarthropathies than in tissues from patients with inactive RA or osteoarthritis and from normal subjects.3 Given the success of TNF blockade in preventing structural damage in RA, some recent studies have focused on the effect of a‐TNF therapies on the soluble or tissue levels of RANKL and OPG.2,4,5 However, to date, there are still no data to indicate the value of the levels of these proteins in predicting the therapeutic response. The aim of the present study was to investigate the utility of serum (s)RANKL and sOPG to predict a therapeutic response to a‐TNF.
Patients and methods
Patients
The characteristics of the patients included in this study are summarised in table 1, and all of them fulfilled the 1988 RA classification criteria.6 Patients treated with adalimumab (ADA) were those enrolled in the clinical trial “ReAct” at our three hospitals, while those treated with infliximab (INF) were consecutive patients from our daily clinical practice. Serum samples were obtained prior to commencing the a‐TNF treatment (baseline), and a second sample (final) was obtained either at week 12 in patients treated with ADA or prior to the 4th infusion (week 14) in those treated with INF. The laboratory and clinical information for each patient was obtained from clinical records at baseline, 3 months (week 12 (ADA) and 14 (INF)) and 7 months (week 28 (ADA) and 30 (INF)). All patients gave their informed written and signed consent.
Table 1 Patient characteristics.
| Total | Adalimumab | Infliximab | p | |
|---|---|---|---|---|
| Number | 75 | 65 | 10 | – |
| Female n (%) | 61 (81.3) | 51 (78.5) | 10 (100) | NS* |
| Age (years) | 56 (14) | 57 (14) | 50 (14) | NS† |
| Disease duration (years) | 8 (4–15) | 8.5 (4–15) | 7.5 (6–24) | NS‡ |
| RF positive n (%) | 65 (86.7) | 58 (89.2) | 7 (70) | NS* |
| DAS28 baseline | 5.9 (1) | 5.9 (1.1) | 6 (1) | NS† |
| HAQ baseline | 1.6 (1.1–2.1) | 1.6 (1.1–2.3) | 1.6 (1.3–2) | NS‡ |
| Ph‐GDA baseline | 61 (16) | 60 (15) | 70 (19) | 0.06† |
| CRP baseline (g/l) | 15 (9–24) | 15 (8–22) | 27 (11–42) | 0.05‡ |
| Clinical response n (%) | 62 (82.7) | 55 (84.6) | 7 (70) | NS* |
| Remission (%) | 10 (13.3) | 8 (12.3) | 2 (20) | NS* |
*χ2 test; †t test; ‡Mann–Whitney test.
RF, rheumatoid factor; DAS28, 28 joint count disease activity score; Ph‐GDA, physician's global disease assessment; CRP, C‐reactive protein.
The response to treatment at the analysed visits was classified as remission (DAS28<2.6), clinical response without remission (a decrease in DAS28⩾1.2 respect baseline visit) and no response (a decrease in DAS28<1.2 respect baseline visit).
Measurement of RANKL and OPG in serum
Serum OPG and RANKL levels were assessed (blind testing) using a commercially available enzyme‐linked immunoabsorbent assay from R&D Systems (Minneapolis, MN) and Immundiagnostik/Apotech (Epalinges, Switzerland), respectively. The coefficients of variation for the intra‐ and interassay were 5.1% and 12.1%, respectively, for OPG and 6.2% and 9.7%, respectively, for RANKL measurements.
Statistical analysis
The statistical analyses were all performed using Stata 9.2 for Windows (StataCorp LP, College Station, TX). The appropriate tests were applied according to the distribution of variables. To estimate the effect of the different variables on the response to treatment, were multivariate logistic regression models was designed including age, gender, baseline DAS28 and physician global disease assessment, as well as sOPG and sRANKL. The final model was reached by using stepwise backward estimation, removing all variables with p>0.05.
Results
Baseline and final serum RANKL and OPG levels
In accordance with earlier data,7,8 we observed that baseline and post‐treatment sOPG levels correlated significantly with age (r = 0.24; p = 0.045) and were lower in males than in females (2197 pg/ml (IQR: 1829–4672) vs 4099 pg/ml (2395–5696), respectively; p = 0.09). In addition, we found a modest but significant correlation between the baseline sOPG levels and both the HAQ score (r = 0.32; p = 0.007) and the physician's global disease assessment (r = 0.35; p = 0.003). However, the correlation of sOPG with DAS28 was not statistically significant (data not shown). Similarly, no significant association between the levels of sRANKL and patient characteristics or disease variables was observed, except for a mild correlation between RANKL and DAS28 (r = 0.3; p = 0.01).
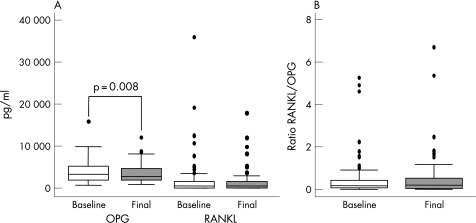
With regards to the effect of TNF blockade, we found that a‐TNF was associated with a decrease in the levels of both sOPG and sRANKL in the overall population, although this reduction was only significant for OPG (fig 1A). Since both sRANKL and sOPG levels diminished after blocking TNF, this therapy failed to produce a significant modification on the serum RANKL/OPG ratio with respect to the baseline (fig 1B).
Figure 1 Serum levels of OPG, RANKL and the RANKL/OPG ratio in a population of rheumatoid arthritis patients treated with antagonists of TNF (a‐TNF). (A) TNF blockade induced a significant downregulation of serum OPG and a decrease in RANKL levels. The data are presented as the interquartile range (p75 upper side of the box, p25 lower side, p50 midline in the box), as well as the p95 (upper line from the box) and p5. Dots represent outliers. (B) The RANKL/OPG ratio is not affected by a‐TNF treatment. The data are shown as described in panel (A). Statistics: Wilcoxon's test for paired samples.
Baseline serum RANKL and RANKL/OPG ratio predicts short‐term remission after TNF blockade
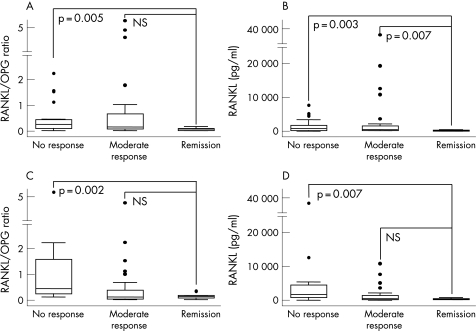
We observed that patients in remission after TNF blockade displayed notably lower levels of both baseline RANKL/OPG ratio and sRANKL than those patients whose DAS28 remained >2.6, independently of obtaining a relevant response or not. These findings were consistent when the response to treatment was evaluated after 3 or 7 months of treatment (fig 2). No significant differences were observed in sOPG baseline levels between these three groups of patients (data not shown).
Figure 2 Relationship between the response to treatment with antagonists of TNF and the serum levels of the RANKL/OPG ratio and RANKL. The baseline levels of RANKL and the RANKL/OPG ratio are lower in patients that achieve remission (defined as DAS28<2.6 at the final visit) than those with partial (final DAS28>2.6 but improved >1.2) or no response (DAS28 improvement<1.2) at 3 months (A and B) or 7 months of follow‐up (C and D). Data are presented as the interquartile range (p75 upper side of the box, p25 lower side, p50 midline in the box), as well as the p95 (upper line from the box) and p5. Dots represent outliers. Statistics: Mann–Whitney test for all panels. Due to multiple comparisons, the significance level was considered if p<0.015.
Since we observed a weak association between the sRANKL levels and the baseline DAS28, we performed a multivariate logistic regression to determine whether the association between remission and low levels of RANKL might have been biased by the baseline DAS28. The best model was fitted with the variables age, gender and baseline DAS28 and sRANKL. Consequently, we found that both lower DAS28 values (OR 0.314 (95% confidence interval: 0.109–0.904), p = 0.032) and sRANKL levels (OR 0.993 (0.987–0.999), p = 0.037) were significantly and independently associated with remission.
On the other hand, none of the variables analysed in this study were able to predict an absence of response to a‐TNF.
Discussion
Prognostic factors and disease markers that can predict the therapeutic response to a‐TNF therapy in patients with RA might be invaluable tools to select the optimum candidates for treatment. The most relevant result in the current study is the finding that both baseline RANKL/OPG ratio and serum RANKL levels may serve to predict remission in patients with established RA treated with a‐TNF.
Interestingly, the possible predictive value of the serum RANKL/OPG ratio to predict radiographic progression in early RA was recently addressed.9 In patients that had not been exposed to disease‐modifying antirheumatic drugs (DMARD), the progression of radiographic damage was greatest in those with a high ESR and a low OPG/RANKL ratio.9 The high levels of RANKL in the inflamed joint of patients with RA are produced by infiltrating activated cells and resident synoviocytes, and it is likely to be an important cause of joint erosion in RA. Indeed, this feature could be linked to a more aggressive disease condition. In this respect, our data show a possible relationship between baseline DAS28 and RANKL levels, although this possible bias was ruled out following the logistic regression analysis. Furthermore, both lower baseline DAS28 values and sRANKL levels were significantly and independently associated with remission. Hence, patients with lower levels of sRANKL, irrespective of their activity score at baseline, obtained the optimal response from a‐TNF therapy.
A possible explanation for our findings is that RANKL expression is regulated by TNF as well as other cytokines.10,11,12 In this regard, it seems feasible that high sRANKL levels may be a consequence of activation through several pathways. In these patients, a‐TNF might promote a therapeutic response but not remission, since pathogenic mechanisms other than TNF may remain active.
The measurement of sRANKL could be useful in those patients whose condition is severe and active, and in whom the efficiency/safety ratio might be “borderline”. In such cases, possessing information to indicate the possibility of achieving an optimal response could sway the clinical decision towards the use of this therapy. Unfortunately, sRANKL cannot discriminate those patients who will not respond.
Pretreatment levels of sOPG did not predict the clinical outcome in our population, although they were correlated with some parameters of active inflammation as described with the ESR in other studies.9,13 As occurs in disorders of elevated bone turnover,7 the upregulation of OPG could be a compensatory mechanism to limit bone erosion. Here, the reduction in sOPG parallels the disease improvement, and similar reductions have been reported previously with infliximab.2 It is conceivable that the reduction in inflammation induced by TNF inhibition could drive a reversion of the upregulation of sOPG associated with inflammatory response.
Finally, TNF blockade is not the only means by which serum and tissue levels of RANKL and OPG can be modified. Indeed, INF, MTX and SSZ each inhibited the expression of RANKL in RA synoviocytes while augmenting the secretion of OPG in synoviocyte supernatants, and they all inhibited osteoclast formation in vitro.5 Therefore, the predictive value of baseline sRANLK levels for a‐TNF therapy must also be evaluated for other therapeutic approaches. Comparative studies with traditional DMARDs or other biological therapies will be warranted to elucidate the potential class‐specificity of our findings.
Acknowledgements
We would like to thank Dr Fernando Gamero for the collection of clinical data from patients in Hospital Universitario La Paz, and Dr Loreto Carmona for critical reading of the manuscript.
Abbreviations
ADA - adalimumab
a‐TNF - TNF antagonists
DAS28 - 28 joint count disease activity score
HAQ - health assessment questionnaire
INF - infliximab
IQR - interquartile range
OPG - osteoprotegerin
RA - rheumatoid arthritis
RANK - receptor activator for nuclear factor kappaB
RANKL - receptor activator for nuclear factor kappaB ligand
Footnotes
Competing interests: None declared.
This work has been funded by Instituto de Salud Carlos III (FIS 04/2009 and 05/2041) and a grant from Abbott Laboratories.
References
- 1.Gravallese E M, Goldring S R. Cellular mechanisms and the role of cytokines in bone erosions in rheumatoid arthritis. Arthritis Rheum 2000432143–2151. [DOI] [PubMed] [Google Scholar]
- 2.Ziolkowska M, Kurowska M, Radzikowska A, Luszczykiewicz G, Wiland P, Dziewczopolski W.et al High levels of osteoprotegerin and soluble receptor activator of nuclear factor kappa B ligand in serum of rheumatoid arthritis patients and their normalization after anti‐tumor necrosis factor alpha treatment. Arthritis Rheum 2002461744–1753. [DOI] [PubMed] [Google Scholar]
- 3.Crotti T N, Smith M D, Weedon H, Ahern M J, Findlay D M, Kraan M.et al Receptor activator NF‐kappaB ligand (RANKL) expression in synovial tissue from patients with rheumatoid arthritis, spondyloarthropathy, osteoarthritis, and from normal patients: semiquantitative and quantitative analysis. Ann Rheum Dis 2002611047–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Catrina A I, af Klint E, Ernestam S, Catrina S B, Makrygiannakis D, Botusan I R.et al Anti‐tumor necrosis factor therapy increases synovial osteoprotegerin expression in rheumatoid arthritis. Arthritis Rheum 20065476–81. [DOI] [PubMed] [Google Scholar]
- 5.Lee C K, Lee E Y, Chung S M, Mun S H, Yoo B, Moon H B. Effects of disease‐modifying antirheumatic drugs and antiinflammatory cytokines on human osteoclastogenesis through interaction with receptor activator of nuclear factor kappaB, osteoprotegerin, and receptor activator of nuclear factor kappaB ligand. Arthritis Rheum 2004503831–3843. [DOI] [PubMed] [Google Scholar]
- 6.Arnett F C, Edworthy S M, Bloch D A, McShane D J, Fries J F, Cooper N S.et al The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 198831315–324. [DOI] [PubMed] [Google Scholar]
- 7.Khosla S, Arrighi H M, Melton L J, 3rd, Atkinson E J, O'Fallon W M, Dunstan C.et al Correlates of osteoprotegerin levels in women and men. Osteoporos Int 200213394–399. [DOI] [PubMed] [Google Scholar]
- 8.Kudlacek S, Schneider B, Woloszczuk W, Pietschmann P, Willvonseder R. Serum levels of osteoprotegerin increase with age in a healthy adult population. Bone 200332681–686. [DOI] [PubMed] [Google Scholar]
- 9.Geusens P P, Landewe R B, Garnero P, Chen D, Dunstan C R, Lems W F.et al The ratio of circulating osteoprotegerin to RANKL in early rheumatoid arthritis predicts later joint destruction. Arthritis Rheum 2006541772–1777. [DOI] [PubMed] [Google Scholar]
- 10.Kotake S, Nanke Y, Mogi M, Kawamoto M, Furuya T, Yago T.et al IFN‐gamma‐producing human T cells directly induce osteoclastogenesis from human monocytes via the expression of RANKL. Eur J Immunol 2005353353–3363. [DOI] [PubMed] [Google Scholar]
- 11.Liu X H, Kirschenbaum A, Yao S, Levine A C. Cross‐talk between the interleukin‐6 and prostaglandin E(2) signaling systems results in enhancement of osteoclastogenesis through effects on the osteoprotegerin/receptor activator of nuclear factor‐{kappa}B (RANK) ligand/RANK system. Endocrinology 20051461991–1998. [DOI] [PubMed] [Google Scholar]
- 12.Sato K, Takayanagi H. Osteoclasts, rheumatoid arthritis, and osteoimmunology. Curr Opin Rheumatol 200618419–426. [DOI] [PubMed] [Google Scholar]
- 13.Hofbauer L C. Pathophysiology of RANK ligand (RANKL) and osteoprotegerin (OPG). Ann Endocrinol (Paris) 200667139–141. [DOI] [PubMed] [Google Scholar]