Presenilin 1 (PSEN1) mutations account for the majority of cases of autosomal dominant early onset Alzheimer's disease (ADEOAD).1,2 The PSEN1 gene encodes for a 467 amino acid transmembrane protein which functions as a subunit of the γ‐secretase complex that cleaves amyloid precursor protein to generate the Aβ amyloid peptide. More than 160 different mutations have been reported (www.molgen.ua.ac.be/ADMutations) with a great diversity of phenotypes: very early age of onset, early myoclonus seizures, parkinsonism, spastic paraplegia associated with “cotton wool plaques” or rare frontotemporal variant of AD. Ataxia has been rarely described during the course of ADEOAD caused by PSEN1 mutations. Ataxia and psychiatric signs were recently reported as initial symptoms associated with a PSEN1 Ser170Phe mutation.3 We report three patients from the same family with early prominent severe ataxia associated with dementia caused by a novel PSEN1 mutation.
Case report
Patient III2
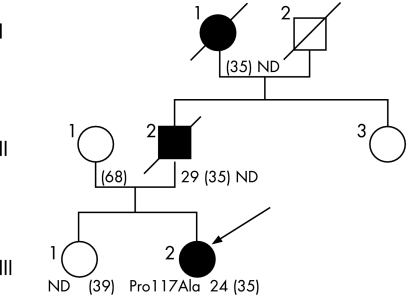
A 35‐year‐old woman (fig 1) was seen for writing and balance difficulties, with memory complaints. All three symptoms occurred contemporaneously 1 year previously. Examination showed severe ataxia with instability, marked dysarthria and dysmetria with intention tremor, brisk reflexes and rare myoclonus. Neuropsychological assessment, including Mini Mental Scale (score 22/30), Grober and Buschke Learning Test (GBVLT) (delayed total recall 5/16), Digits Backwards (3), Backward Spatial Span (3), Trail Making Test Task A (time 104 s) and B ( = 191 s), Rey–Osterrieth Complex Figure Copy (18/36) and 2 min category fluency task (n = 13 animals) yielded abnormal performances. Cerebral MRI revealed mild cortical atrophy. CSF examination, including 14.3.3 protein analysis, was normal. CSF amyloid β (Aβ) levels were not investigated. EEG detected sporadic bilateral generalised spikes and waves that were asymptomatic. Spinocerebellar ataxias types 1, 2, 3, 6, 7 and 17, and dentato‐rubro‐pallido‐luysian atrophy were excluded. Sequence analysis of the prion protein gene was normal. Two years later the patient was completely dependent, with worsening of balance difficulties and falls twice per month. Cognitive state had deteriorated, with severe GBVLT immediate recall impairment (2/16), severe visuocontructive deficit (Rey figure copy 6/36) and reduced verbal fluency (six animals in 2 min).
Figure 1 Family pedigree. Squares = males; circles = females; filled symbols = affected subjects; diagonal line = deceased subject; arrow = proband. ND, sequencing not done. Age of onset, current age or age at death (in parentheses) are indicated.
A second MRI showed a diffuse cerebral atrophy, and Tc‐99m HMPAO SPECT found hypoperfusion in the associated parietal area without cerebellar hypoperfusion.
Patient II2
At age 29 years, the father of patient III2 (fig 1) had developed cerebellar signs with head tremor associated with upper limb extremities intention tremor, dysarthria and gait difficulties. Examination at age 32 years displayed cerebellar signs with dysmetria, gait ataxia and left Babinski sign. EEG, CSF study and fractionated gas encephalography were normal. Examination at age 35 years demonstrated severe axial and four limb ataxia, dysarthria and pyramidal signs with hyperreflexia and lower limb spasticity. Impairment of short term and long term memory was noted but no neuropsychological test was performed. Cerebral tomodensitometry was normal. Patient status worsened rapidly and he died a few months later. Autopsy was not done.
Patient I1
Patient II2's mother complained of gait instability from the age of 24 years (fig 1). Examination revealed gait ataxia and balance impairment with wide based gait, four limb dysmetria and dysarthria. Subsequently, memory deterioration appeared as well as pyramidal signs. Cerebellar signs and cognitive impairment progressively worsened and the patient was then lost to follow up. She died at age 35 years.
Genomic DNA was isolated from blood lymphocytes of patient III2 and her mother II1 after informed consent was obtained. The entire coding sequence and the exon/intron boundaries of the PSEN1 gene were sequenced, as previously described.4 To ensure that mutations detected in patients were not common polymorphisms, we determined that they were absent in 50 control DNA samples.
One novel PSEN1 mutation was found in patient III2. She was heterozygous for the c349 C>G pPro117Ala mutation in exon 5 and for the known polymorphism c953 A>G pGlu318Gly in exon 9. No PSEN1 mutation was found in II1. This was consistent with the inheritance of both the mutation and polymorphism of patient III2 from her father.
Discussion
We have reported three patients from the same family affected with early progressive ataxia and dementia, associated with a new PSEN1 mutation. PSEN1 mutations account for 50–60% of ADEOAD.4,5,6 Cerebellar signs, that occurred 1–7 years after the onset of cognitive deterioration, were previously reported with the following PSEN1 mutations: Met139Val,7 Glu280Ala,8 Leu166Pro,9 Tyr256Ser10 and Leu282Val.11 Our description seems similar to the predominant cerebellar ataxia associated with psychiatric symptoms recently reported in a 28‐year‐old‐man carrying a PSEN1 Ser170Phe mutation.3 Of note, the phenotype was homogeneous in our family with early onset before 35 years and death between 35 and 40 years. This severity can be correlated with the Pro117Ala genotype because other substitutions of proline in position 117 (Pro117Leu, Pro117Arg, Pro117Ser) have been described previously as responsible for severe ADEOAD. However, no cerebellar sign was associated with these mutations. The original phenotype in the present family is due to the Pro117Leu mutation because the associated Glu318Gly substitution in exon 9 is a non‐causative polymorphism.12
Whether ataxia is correlated with cerebellar pathology was not demonstrated in the present case in the absence of a neuropathological study. Cerebellar changes such as Aβ deposition in the molecular and inner granular layers and amyloid angiopathy have been reported in a series of 48 PSEN1 linked ADEOAD but none had cerebellar ataxia.8 Conversely, severe cerebellar degeneration was reported in patients who presented with cerebellar signs during the course of AD due to an Ile143Thr mutation.13 Moreover, abundant diffuse amyloid deposits in the molecular layer, plaques in Purkinje cells and inner granular layers and severe loss of Purkinje cell dendrites were demonstrated in an ataxic case with a PSEN1 Ser170Phe mutation.3
In conclusion, the present study demonstrates that PSEN1 linked ADEOAD has to be considered, even when ataxia precedes dementia, the Pro117Ala mutation being responsible for a predominant precocious ataxia. Correlations between the functional consequences of this novel mutation on the Aβ species and this ataxic variant of AD are still not understood.
Footnotes
Competing interest: None
References
- 1.Sherrington R, Rogaev E I, Liang Y.et al Cloning of a gene bearing missense mutations in early‐onset familial Alzheimer's disease. Nature 1995375754–760. [DOI] [PubMed] [Google Scholar]
- 2.Rogaev E I, Sherrington R, Rogaeva E A.et al Familial Alzheimer's disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer's disease type 3 gene. Nature 1995376775–778. [DOI] [PubMed] [Google Scholar]
- 3.Piccini A, Zanusso G, Borghi R.et al Association of a presenilin 1 S170F mutation with a novel Alzheimer disease molecular phenotype. Arch Neurol 200764738–745. [DOI] [PubMed] [Google Scholar]
- 4.Raux G, Guyant‐Marechal L, Martin C.et al Molecular diagnosis of autosomal dominant early onset Alzheimer's disease: an update. J Med Genet 200542793–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hutton M, Busfield F, Wragg M.et al Complete analysis of the presenilin 1 gene in early onset Alzheimer's disease. Neuroreport 19967801–805. [DOI] [PubMed] [Google Scholar]
- 6.Arango D, Cruts M, Torres O.et al Systematic genetic study of Alzheimer disease in Latin America: mutation frequencies of the amyloid beta precursor protein and presenilin genes in Colombia. Am J Med Genet 2001103138–143. [DOI] [PubMed] [Google Scholar]
- 7.Finckh U, Muller‐Thomsen T, Mann U.et al High prevalence of pathogenic mutations in patients with early‐onset dementia detected by sequence analyses of four different genes. Am J Hum Genet 200066110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mann D M, Pickering‐Brown S M, Takeuchi A.et al Amyloid angiopathy and variability in amyloid beta deposition is determined by mutation position in presenilin‐1‐linked Alzheimer's disease. Am J Pathol 20011582165–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moehlmann T, Winkler E, Xia X.et al Presenilin‐1 mutations of leucine 166 equally affect the generation of the Notch and APP intracellular domains independent of their effect on Abeta 42 production. Proc Natl Acad Sci U S A 2002998025–8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miklossy J, Taddei K, Suva D.et al Two novel presenilin‐1 mutations (Y256S and Q222H) are associated with early‐onset Alzheimer's disease. Neurobiol Aging 200324655–662. [DOI] [PubMed] [Google Scholar]
- 11.Dermaut B, Kumar‐Singh S, De Jonghe C.et al Cerebral amyloid angiopathy is a pathogenic lesion in Alzheimer's disease due to a novel presenilin 1 mutation. Brain 20011242383–2392. [DOI] [PubMed] [Google Scholar]
- 12.Mattila K M, Forsell C, Pirttila T.et al The Glu318Gly mutation of the presenilin‐1 gene does not necessarily cause Alzheimer's disease. Ann Neurol 199844965–967. [DOI] [PubMed] [Google Scholar]
- 13.Martin J J, Gheuens J, Bruyland M.et al Early‐onset Alzheimer's disease in 2 large Belgian families. Neurology 19914162–68. [DOI] [PubMed] [Google Scholar]