Abstract
Objective
To assess both feasibility and short term outcomes of a population based colorectal cancer screening programme using a biennial guaiac based faecal occult blood test (gFOBT).
Method
All participants were invited by mail to take part in a screening programme using a non‐rehydrated gFOBT. The gFOBTs were first provided by general practitioners (GPs) and then directly mailed to individuals who failed to comply after two invitations. The setting was a French administrative district: Haut‐Rhin (710 000 inhabitants). 182 981 residents aged 50–74 years were invited to participate.
Results
19 274 people (10.5%) were excluded from gFOBT screening and 90 706 completed a gFOBT, so that the participation rate was 55.4% of those eligible. 76.5% of the completed gFOBTs were provided by GPs and 15.5% by direct mailing. The gFOBT positivity rate was 3.4%. The positive predictive value was 42.7% for neoplasia (women 30.8%, men 52.5%), 23.6% for advanced adenoma, and 7.6% for cancer. The number of normal colonoscopic procedures (without neoplasia) needed to be performed for each colonoscopy detecting an advanced neoplasia was 1.8, lower in men (1.2) than in women (3.4), and decreasing with age. Detection rates for neoplasia and cancer were 12.8 and 2.3 per 1000 people screened. 206 adenocarcinomas were detected: 47.6% were stage I and 23.8% stage II. The direct cost was estimated at €29.3 per screened person and €13 466 per cancer detected.
Conclusions
Participation and diagnostic yield of controlled trials of gFOBT screening are reproducible in the real world at an acceptable cost through an organised population based programme involving GPs.
Keywords: colorectal neoplasms, screening, occult blood
Colorectal cancer (CRC) is the second most common cause of death from malignant disease in France and resulted in about 16 000 deaths in 2000.1 Randomised controlled trials have demonstrated the efficacy of screening with the guaiac based faecal occult blood test (gFOBT) on both CRC mortality and incidence.2,3,4,5 The European controlled trials using a biennial non‐rehydrated gFOBT showed reductions of 15% to 18% in deaths from CRC after screening.4,5,6 These trials were conducted by highly motivated research teams. Whether these results are transposable in the real world is questionable. In 2002, France initiated a demonstration pilot trial in 23 areas (fig 1) to assess both feasibility and effectiveness of a population based CRC screening programme using a biennial gFOBT.
Figure 1 Twenty‐three pilot trial areas in France, Haut‐Rhin is the darkly shaded area.
We report the short term outcomes of the first round of the screening programme conducted in the administrative district of the Haut‐Rhin in eastern France, one of the 23 pilot areas.
Methods
The Haut‐Rhin is an area with a population of around 710 000 inhabitants. The screening programme was conducted according to national specifications adapted from the experience acquired with the French controlled trial conducted in Burgundy.6,7
Residents aged 50–74 were invited to participate. They were identified using the Sickness Fund database files. A first letter was mailed inviting them to visit their general practitioner (GP) for a CRC screening. Six months later, a first recall letter was mailed to all those who had not visited their GP. This recall letter included a reply coupon, which could be used to specify reasons for non‐participation. Four months later, gFOBTs were directly mailed with instructions for use to people who had not complied. A last recall letter was mailed six weeks later if necessary.
GPs were asked to exclude from screening with gFOBT any person with serious illness, with recent CRC screening (FOBT <2 years or colonoscopy <5 years) or with high CRC risk (family history of CRC in a first degree relative aged <60 years or in two first degree relatives; personal history of colorectal adenoma or cancer or inflammatory bowel disease colitis). GPs were also asked to convince people with average risk to participate and to provide the gFOBTs free of charge in addition to explaining the instructions for use.
The gFOBT (Hemoccult II) was used without dietary restriction. The only restriction was the use of >1 g of acetylsalicylic acid and vitamin C. Faecal material was assessed from two samples from each of three consecutive stools. Completed gFOBTs were sent to a central laboratory in Mulhouse (Centre d'Examens de Santé de la Caisse Primaire d'Assurance Maladie) where they were processed without rehydration. The test was defined as positive as soon as one slide was positive. Test results were mailed both to the participants and their GPs. People who had a positive test result were instructed to visit their GP who would prescribe a colonoscopy. Colonoscopies were performed by the 34 gastroenterologists practising in the Haut‐Rhin. Histopathological examinations of biopsy, polypectomy, and resection specimens were carried out by the 14 general pathologists practising in the Haut‐Rhin.
The result of each colonoscopy was classified according to the lesion with the worst prognosis. Cancer was defined as carcinoma invading at least the submucosa across the muscularis mucosa (category 5 in the revised Vienna classification of gastrointestinal epithelial neoplasia).8 In situ and intramucosal carcinomas (categories 4.2 and 4.4 in the revised Vienna classification) were classified as high grade dysplasia. Cancers were classified according to the tumour‐node‐metastasis (TNM) and American Joint Committee on Cancer (AJCC) classifications.9,10,11 Adenomas treated by endoscopic polypectomy and found to contain invasive cancer were classified as stage I. Advanced adenoma was defined as an adenoma ⩾10 mm or with a villous component >20% or with high grade dysplasia. Advanced neoplasia was defined as a cancer or an advanced adenoma.
The direct costs were estimated in 2006 euros through the budget of the screening programme. Direct medical costs related to management of gFOBT positive participants were excluded, in particular the fees related to colonoscopies.
The χ2 test was used for statistical analysis. The significance threshold was set at 0.05.
Results
Participation
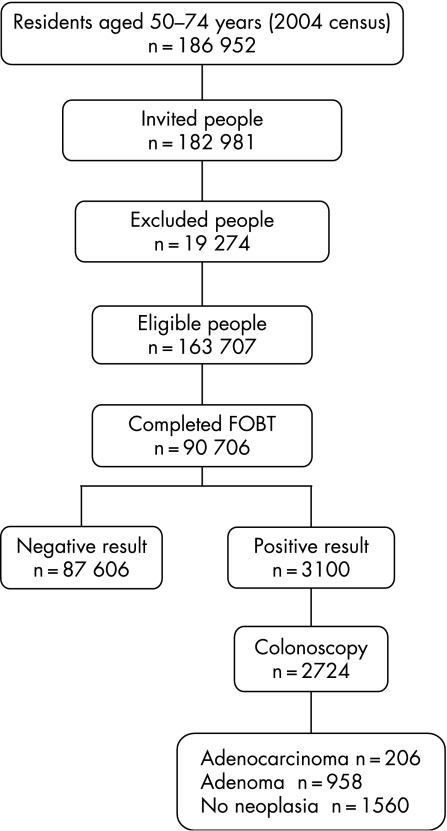
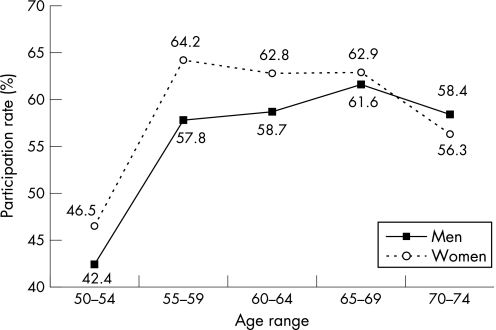
The first round of the programme lasted from September 2003 to September 2006. According to the 2004 census population (www.insee.fr), 186 952 people aged 50–74 years live in the Haut‐Rhin. Figure 2 details participant numbers throughout the pilot programme: 182 981 people (97.9%) were invited to participate. Their age and sex distributions are listed in table 1. A total of 19 274 people (10.5%) were excluded so that 163 707 were eligible for gFOBT screening. The main reasons for exclusion were recent CRC screening (6.8%), high CRC risk (3.6%), and serious illness (0.5%). The main sources of information leading to exclusion were the reply coupon (46.1%) and the GPs (41.2%). A final (positive or negative) test result was available for 90 706 people who completed a gFOBT so that the participation rate was 55.4% of eligible individuals. The participation rate was significantly higher in women (57.0%) than in men (53.7%) (p<0.001) and in those aged 55–69 years (61.3%) than in the younger (44.5%) and older (57.2%) age groups (fig 3). The participation rate was lower in urban areas (53.5%) than in rural (56.7%) and suburban areas (58.9%) (p<0.001).
Figure 2 Flow diagram of the pilot programme.
Table 1 Age and sex distributions of people invited to participate.
| Age (years) | Men No (%) | Women No (%) |
|---|---|---|
| 50–54 | 26 963 (30.6) | 27 943 (29.4) |
| 55–59 | 19 106 (21.7) | 18 849 (19.9) |
| 60–64 | 15 591 (17.7) | 15 909 (16.8) |
| 65–69 | 13 834 (15.7) | 15 893 (16.7) |
| 70–74 | 12 549 (14.3) | 16 344 (17.2) |
| Total | 88 043 | 94 938 |
Figure 3 Participation rates according to age and sex.
GP involvement
A total of 618 GPs (98.6%) participated and provided a mean of 135 gFOBTs (range 1–671); 81.6% of people who received a gFOBT from their GP actually completed it. Overall, 76.5% of the completed gFOBTs were provided by GPs and 15.5% by direct mailing; 5.4% were provided during a health check‐up and 1% by occupational medicine physicians. The first invitation letter had an impact for about 6 months and accounted for 50.1% of the completed gFOBTs. The impact of the first recall letter lasted 4 months and accounted for 26.1% of the completed gFOBTs. The gFOBT was directly mailed to 89 365 people (47.8% of the population) and was actually completed by 16.3% of them.
gFOBT results
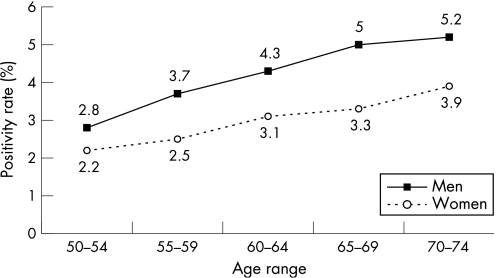
The overall positivity rate was 3.4%. It was higher in men (4.0%) than in women (2.9%) and increased with age (fig 4). The result of 2992 gFOBTs (3.2%) was doubtful so that the test had to be completed again; 2315 (77.4%) were actually completed again. The number of positive slides per test was one or two in 67.1% of cases and three or four in 23.0% of cases.
Figure 4 Positivity rates according to age and sex.
Colonoscopy
A total of 2724 colonoscopies (87.9%) were performed in people with a positive gFOBT (1492 in men and 1232 in women). The rate of completed colonoscopies was significantly lower in the 70–74 year age group (84.9%) than in the younger age group (88.6%) (p = 0.02), with no difference by sex. The caecal intubation rate was 95.0%. Six (0.2%) serious complications were reported: two perforations requiring surgery and four episodes of bleeding requiring an additional endoscopic procedure for haemostasis. One of the two perforations occurred during endoscopic mucosal resection of a 30 mm sessile adenoma of the right colon that would have required surgical removal if the endoscopic resection had not been performed. Nine people (0.3%) were admitted for overnight observation because of minor bleeding or abdominal pain.
Diagnostic yield
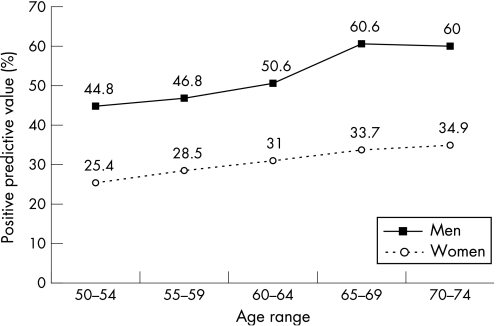
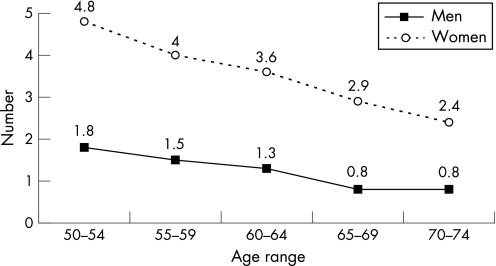
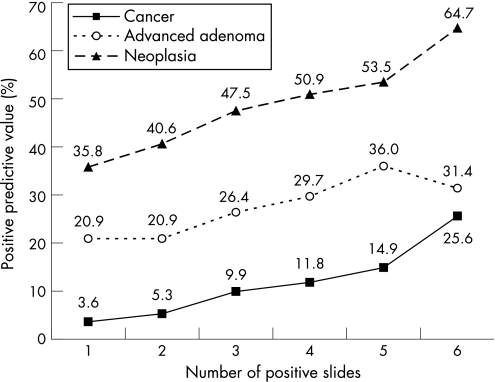
There were 51.2% of the colonoscopies shown to be abnormal, with either cancers or polyps being detected. The positive predictive value was 7.6% for cancers, 23.6% for advanced adenomas (2.9% for in situ carcinomas), and 11.5% for non‐advanced adenomas. The positive predictive value for neoplasia was 42.7%, higher in men (52.5%) than in women (30.8%) and increased with age (fig 5). The number of colonoscopic procedures needed to detect advanced neoplasia was 3.2, twofold higher in women (4.9) than in men (2.5) and decreasing with age. The number of normal colonoscopic procedures (without neoplasia) to perform for each colonoscopy detecting an advanced neoplasia was 1.8, lower in men (1.2) than in women (3.4) and decreasing with age (fig 6). The positive predictive value for cancer and for advanced adenoma increased with the number of positive slides (fig 7). In all, 2491 polyps were detected, 80.3% of them were adenomas; 26.1% of the adenomas measured 10–19 mm and 16.8% were larger than 20 mm; 58.7% of them were sessile, 35.1% pedonculated, and 6.2% were flat; 68.7% of them were tubular, 24.6% tubulovillous, 2.5% villous, and 4.2% were serrated; 61.0% of the adenomas displayed low grade dysplasia, 36.3% high grade dysplasia (4.6% with in situ carcinoma), and 2.7% invasive cancer; 1721 benign adenomas (96.1%) were removed endoscopically and 67 had to be removed surgically in 50 people.
Figure 5 Positive predictive values for neoplasia according to age and sex.
Figure 6 Number of normal colonoscopic procedures needed to be performed for each colonoscopy detecting an advanced neoplasia according to age and sex.
Figure 7 Positive predictive values for neoplasia according to the number of positive slides per test.
Neoplasia detection rates and number needed to screen
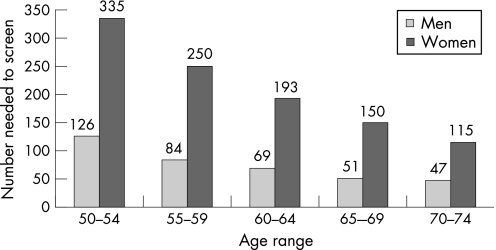
The cancer detection rate was 2.3 per 1000 people screened, higher in men (3.2 per 1000) than in women (1.4 per 1000). The neoplasia detection rate was 12.8 per 1000 people screened. It was higher in men (18.5 per 1000) than in women (7.9 per 1000) and increased with age. The number needed to screen to detect advanced neoplasia was 107, 2.7 times higher in women (191) than in men (71) and decreasing with age (fig 8).
Figure 8 Number needed to screen to detect advanced neoplasia according to age and sex.
Screen detected cancers
Overall, 207 cancers were detected by screening. There were 206 adenocarcinomas and one lymphoma. Two people died before surgery, one was lost to follow‐up and 18 rectal cancers were treated with radiotherapy and/or chemotherapy before surgery, so that their initial TNM stage could not be assessed. The TNM and AJCC stage distributions of 185 screen detected adenocarcinomas are listed in table 2. In all, 18.5% of the adenocarcinomas were located in the rectum, 52.4% in the distal colon, and 29.1% in the proximal colon; 49 adenocarcinomas (23.8%) were polyp cancers, of which 19 (38.8%) were exclusively treated by endoscopic resection.
Table 2 AJCC and TNM stages of 185 adenocarcinomas detected by screening.
| AJCC stage | (TNM stage) | No (%) |
|---|---|---|
| I | (T1–T2 N0 M0) | 88 (47.6) |
| II | (T3–T4 N0 M0) | 44 (23.8) |
| IIa | (T3 N0 M0) | 40 (21.6) |
| IIb | (T4 N0 M0) | 4 (2.2) |
| III | (Any T N1 M0) | 38 (20.5) |
| IIIa | (T1–T2 N1 M0) | 5 (2.7) |
| IIIb | (T3–T4 N1 M0) | 20 (10.8) |
| IIIc | (Any T N2 M0) | 13 (7.0) |
| IV | (Any T any N M1) | 15 (8.1) |
Cost
The annual budget of this programme was €1.229 million. The direct cost per screened individual was €29.3. It could be split into a fixed cost of €4.9 a year per 50–74‐year‐old individual (for logistics, invitations, purchase of gFOBT, etc), and a variable cost of €7.5 per screened individual (for gFOBT processing, GP retribution, follow‐up after positive test, etc). The cost per cancer detected was €13 466, per advanced neoplasia €3645, and per neoplasia €2162.
Discussion
Our results show that a population based screening programme for CRC using gFOBT is feasible in France at an acceptable cost by involving GPs. The participation of the average risk population and the diagnostic yield of the European controlled trials using a biennial gFOBT are reproducible in the real world. Our participation rate of 55.4% is the highest achieved in the 23 French pilot trial areas (www.invs.sante.fr). It was within the range of rates achieved by the first rounds of the European trials (52.8%–67.2%).4,5,6 The demonstration pilot conducted in the United Kingdom with the same objective as our programme achieved a similar participation rate of 56.8% without any real involvement of the GPs.12 However, GP involvement was the cornerstone of our programme. GPs were the source of three quarters of the participants. Their role was essential for assessing people's CRC risk and comorbidities and for convincing people with average risk to participate. Two previous trials demonstrated that the involvement of GPs is necessary to achieve a high participation rate in France.7,13 As in other CRC screening programmes, women were more likely to comply than men.5,6,12 To achieve a participation rate close to the Danish trial, efforts should be made to increase the participation of men and those under the age of 55 years.4 The involvement of occupational medicine physicians should probably be necessary to convince younger people to participate.
The population used to calculate our participation rate was the eligible average risk population—that is, the invited population minus the population excluded from gFOBT screening. The other European trials used the same approach but their excluded population was far less extensive than ours4,5,6,12; 10.5% of our 50–74‐year‐old population were excluded from gFOBT screening, two‐thirds because they had had a recent CRC screening and one‐third because of a high risk of CRC indicating direct colonoscopy screening. In the European trials and the British pilot, people were only excluded if they had previous CRC or serious illness.4,5,6,12 Exclusion of people with a family history of CRC or with a recent CRC screening was not mentioned in these trials. Exclusions from gFOBT screening can still be increased. Two additional sources of exclusion will be used for the second round of our programme: pathologists will exclude all newly diagnosed CRC cases and gastroenterologists will exclude for a 5 year period all people having a full colonoscopic procedure for whatever reason. Raising the exclusion rate should reduce fixed costs (recall letters and tests for direct gFOBT mailing). Moreover, it should indirectly increase the participation rate by reducing the size of the eligible average risk population.
Our short term outcomes were very similar to the results of the European controlled trials and the British pilot trials except for the gFOBT positivity rate and the CRC detection rate, which were higher.4,5,6,12 Our positivity rate was 3.4% compared with 1–2.1% in the other trials. A quality assessment programme was conducted in the central laboratory where the gFOBTs were processed so that we could exclude problems in the reading process. Moreover our positive predictive value for neoplasia was 42.7%, similar to the other trials (37.3%–49%) and our cancer detection rate was 2.3 per 1000 people screened, slightly higher than the other trials (1.6 to 2.1). Our higher positivity rate was therefore not caused by false positive results but more probably by the high CRC incidence in the Haut‐Rhin. Data from the European cancer registries show that the incidence of CRC in Haut‐Rhin is one of the highest in Europe, in both men and women (www.arer68.fr).
As in the Danish and French trials, the test was defined as positive as soon as one slide was positive.4,6 We did not utilise a repeat test in case of weak positive gFOBT (one to four positive slides) as did the British trials for three reasons5,12: (1) the positive predictive value was far from negligible in the so called “weak positive” tests (fig 7); (2) the main drawback of gFOBT is its low sensitivity and the repeat test would improve specificity to the detriment of sensitivity; and (3) that would lead to a 90.1% rate of repeat tests with compliance problems.
Our positive predictive value for cancer was 7.6%, slightly lower than in the other trials (9.8%–17%).4,5,6,12 This could be partly because of a difference in cancer definition. Whether in situ and intramucosal carcinomas were included in cancers was not specified in the previous French trial.6 Including in situ and intramucosal carcinomas as cancers, our positive predictive value for cancer would increase to 10.5%.
The proportion of screen detected adenocarcinomas presenting at AJCC stages I and II was 71.4%, similar to the figures of the European trials (71–77%).4,5,6,12 This high proportion compares favourably with the 46–52% proportion of early stages in the control groups without screening in the same trials.4,5,6 There were only four stage IIb cancers so that the proportion of adenocarcinomas with a more than 80% 5 year survival rate (stages I, IIa and IIIa) remained high (71.9%).14
It is too early to assess the impact of our programme on CRC mortality and incidence. This assessment will be possible in a few years thanks to the Haut‐Rhin cancer registry. Meanwhile we can only extrapolate from our short term findings: as outcomes were similar to those reported in previous trials, it would be reasonable to expect a reduction of CRC mortality and incidence of comparable magnitude.
The number needed to explore by colonoscopy to detect one advanced neoplasia was 3.2. This confirms that a positive gFOBT is an indication for colonoscopy with one of the highest, if not the highest, yields.15,16 All other screening tools have a lower yield: the number needed to explore by colonoscopy to detect one advanced neoplasia is around 17 for colonoscopy screening17 and around five for flexible sigmoidoscopy screening.18 The number of normal colonoscopic procedures to perform for each colonoscopy detecting an advanced neoplasia was 1.8. This ratio is, in our opinion, the best indicator of the benefit:risk ratio of a CRC screening strategy.16 It reflects both the yield of the screening strategy and its actual cost in terms of potential adverse events and resources requirement. This ratio is favourable in both screening strategies with gFOBT and flexible sigmoidoscopy. It was 2.3 in the PLCO cancer screening trial.18 However, this ratio is prohibitive in a screening strategy with colonoscopy: it was 15.2 in the 50–66 year age group without family history of CRC in the Polish trial.17
The number needed to screen to detect advanced neoplasia was 2.7 times higher in women than in men. These results confirm that there are important differences in CRC risk based on sex. Some authors advocate customising the CRC screening recommendations based on sex.17,19 We agree with a customisation for opportunistic endoscopic screening, either with flexible sigmoidoscopy or with colonoscopy. On the other hand, our data do not favour such a sex specific strategy for mass screening with gFOBT. The question is whether we should initiate mass screening later in women at the age of 55 or even 60 years? If we had adopted these age limits in our programme, seven or even 15 women with CRC (36 or even 79 women with advanced neoplasia) would have been missed. The number needed to screen to detect advanced neoplasia in the 50–54 year age group was indeed high in women (335), 2.7 times higher than in men (126), but this sex ratio was almost the same in all the age groups. Moreover, data from the Haut‐Rhin cancer registry show that the CRC incidence rates in 1997–9 in the 50–54 year age group was 55.3 per 100 000 in men, only a little higher than in women (52.7 per 100 000) (www.arer68.fr). Likewise estimated CRC incidence rates in France in 2000 were in the 50–54 year age group 52.3 per 100 000 in men and 41.4 in women (www.invs.sante.fr/estimations‐cancer). The sex ratio was only 1.3 in this age group, rising with age and reaching 1.9 in the 70–74 year age group. Thus, we should not postpone initiation of screening in women 5–10 years later than in men. Should we initiate screening earlier in men at the age of 45 years? That would lead to participation problems as younger men are less likely to comply than older individuals.
Our 0.2% rate of serious complications was towards the lower end of the range of previously reported rates.20 Our 0.07% rate of perforation was similar to the 0.05% rate of the British pilot.12 Adverse effects of CRC screening with FOBT have not yet been assessed as thoroughly as breast cancer screening with mammography.21 The Danish trial did not mention adverse events4 and the French trial did not observe a single serious complication out of 1691 colonoscopic procedures.6 In fact, the British trial reported a 0.5% rate of serious complications.22 That is one of the reasons why additional real world data are needed to truly assess the risks and benefits of widespread CRC screening with FOBT. Our rates are probably an underestimate as we did not conduct a systematic search for complications. Late and minor complications may have been under‐reported.16,23
Our costs, estimated at €13 466 per cancer detected and €29.3 per screened person, were high compared with previously reported costs. Two studies have assessed the cost of a community based CRC screening programme with gFOBT.24,25 The total cost per cancer detected in an Australian programme was $A28 679 (1995 $A).24 If indirect costs and direct medical costs related to management of FOBT positive participants were excluded, it would be equivalent to €11 831 (2006 €) adjusted for inflation. Using the same calculation method in the first round of the Nottingham trial, the costs were £5072 per cancer detected and £10.9 per screened person (2002 £), equivalent to €8408 and €18.1 (2006 €) adjusted for inflation.25 On the other hand, our costs were quite favourable compared with those of breast cancer screening in France, which were between €11 600 and €13 850 per cancer detected (equivalent to €13 351–€15 921 (2006 €) adjusted for inflation) and between €58 and €60 per woman screened (equivalent to €67 to €69 (2006 €) adjusted for inflation).26 These figures are only “order of magnitude” estimates but they should be helpful for decision making regarding the further implementation of CRC screening in France.
Conclusion
Our results confirm that the guaiac based FOBT, though not the ideal CRC screening tool, is the ideal tool for the immediate widespread implementation of an organised CRC screen. Along with the results of the other pilot areas, they have led the French Health authorities to decide to extend this gFOBT CRC screening programme to the entire nation from 2007 onwards. The gFOBT is not perfect, but it is the only screening tool with high quality evidence obtained from randomised controlled trials demonstrating its efficacy to reduce the risk of death from CRC. Moreover, with less than 5% of the screened population explored by colonoscopy, it is the only mass screening tool compatible with the existing colonoscopic capabilities. The final results of the randomised controlled trials on CRC screening with flexible sigmoidoscopy are awaited.18,27,28 But, whatever their results on CRC mortality, flexible sigmoidoscopy is accepted less well than the gFOBT, except in Scandinavian countries.29 The next step after the widespread implementation of an organised CRC screening with gFOBT will be the improvement of the screening tool, probably by replacing the guaiac based FOBT with an immunochemical FOBT30 and/or its association with flexible sigmoidoscopy.31,32 A trial is in progress in a district of the Haut‐Rhin to assess the feasibility of an organised CRC screening programme combining gFOBT and flexible sigmoidoscopy.
Acknowledgements
The authors thank the GPs of the Haut‐Rhin for their active participation in this pilot programme. They also thank all the staff of ADECA 68 (Association pour le dépistage du cancer colorectal dans le Haut‐Rhin) and the participating gastroenterologists and pathologists for their contributions.
Abbreviations
AJCC - American Joint Committee on Cancer
CRC - colorectal cancer
gFOBT - guaiac based faecal occult blood test
GP - general practitioner
TNM - tumour‐node‐metastasis
Footnotes
Funding: Financing was ensured through the French Sickness Fund (Assurance Maladie) and the Haut‐Rhin Administration (Conseil Général du Haut‐Rhin).
Conflict of interest: None.
References
- 1.Remontet L, Estève J, Bouvier A M.et al Cancer incidence and mortality in France over the period 1978‐2000. Rev Epidemiol Sante Publique 2003513–30. [PubMed] [Google Scholar]
- 2.Mandel J S, Bond J H, Church T R.et al Reducing mortality from colorectal cancer by screening for fecal occult blood. N Engl J Med 19933281365–1371. [DOI] [PubMed] [Google Scholar]
- 3.Mandel J S, Church T R, Bond J H.et al The effect of fecal occult‐blood screening on the incidence of colorectal cancer. N Engl J Med 20003431603–1607. [DOI] [PubMed] [Google Scholar]
- 4.Kronborg O, Fenger C, Olsen J.et al Randomised study of screening for colorectal cancer with faecal‐occult‐blood. Lancet 19963481467–1471. [DOI] [PubMed] [Google Scholar]
- 5.Hardcastle J D, Chamberlain J O, Robinson M H E.et al Randomised controlled trial of faecal‐occult‐blood screening for colorectal cancer. Lancet 19963481472–1477. [DOI] [PubMed] [Google Scholar]
- 6.Faivre J, Dancourt V, Lejeune C.et al Reduction in colorectal cancer mortality by fecal occult blood screening in a French controlled study. Gastroenterology 20041261674–1680. [DOI] [PubMed] [Google Scholar]
- 7.Tazi M A, Faivre J, Dassonville F.et al Participation in faecal occult blood screening for colorectal cancer in a well defined French population: results of five screening rounds from 1988 to 1996. J Med Screen 19974147–151. [DOI] [PubMed] [Google Scholar]
- 8.Schlemper R J, Kato Y, Stolte M. Diagnostic criteria for gastrointestinal carcinomas in Japan and Western countries: proposal for a new classification system of gastrointestinal epithelial neoplasia. J Gastroenterol Hepatol 200015G49–G57. [DOI] [PubMed] [Google Scholar]
- 9.Sobin L H, Wittekind C. eds. TNM classification of malignant tumours.UICC 6th ed. New York: Wiley‐Liss, 2002
- 10.AJCC cancer staging manual. 5th ed. New York: Springer, 1997
- 11.AJCC cancer staging manual. 6th ed. New York: Springer, 2002
- 12.UK Colorectal Cancer Screening Pilot Group Results of the first round of a demonstration pilot of screening for colorectal cancer in the United Kingdom. BMJ 2004329133–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Launoy G, Herbert C, Vallée J P.et al Le dépistage de masse du cancer colorectal en France. Expérience auprès de 165000 personnes dans le Calvados. Gastroenterol Clin Biol 199620228–236. [PubMed] [Google Scholar]
- 14.O'Connell J B, Maggard M A, Ko C Y. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst 2004961420–1425. [DOI] [PubMed] [Google Scholar]
- 15.Lieberman D A, de Garmo P L, Fleischer D E.et al Colonic neoplasia in patients with nonspecific GI symptoms. Gastrointest Endosc 200051647–651. [DOI] [PubMed] [Google Scholar]
- 16.Denis B, Weiss A M, Peter A.et al Quality assurance and gastrointestinal endoscopy: an audit of 500 colonoscopic procedures. Gastroenterol Clin Biol 2004281245–1255. [DOI] [PubMed] [Google Scholar]
- 17.Regula J, Rupinski M, Kraszewska E.et al Colonoscopy in colorectal‐cancer screening for detection of advanced neoplasia. N Engl J Med 20063551863–1872. [DOI] [PubMed] [Google Scholar]
- 18.Weissfeld J L, Schoen R E, Pinsky P F.et al Flexible sigmoidoscopy in the PLCO cancer screening trial: results from the baseline screening examination of a randomized trial. J Natl Cancer Inst 200597989–997. [DOI] [PubMed] [Google Scholar]
- 19.Lieberman D. Race, gender, and colorectal cancer screening. Am J Gastroenterol 20051002756–2758. [DOI] [PubMed] [Google Scholar]
- 20.Waye J D, Kahn O, Auerbach M E. Complications of colonoscopy and flexible sigmoidoscopy. Gastrointest Endosc Clin N Am 19966343–377. [PubMed] [Google Scholar]
- 21.Feig S A. Adverse effects of screening mammography. Radiol Clin N Am 200442807–819. [DOI] [PubMed] [Google Scholar]
- 22.Robinson M H E, Hardcastle J D, Moss S M.et al The risks of screening: data from the Nottingham randomised controlled trial of faecal occult blood screening for colorectal cancer. Gut 199945588–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zubarik R, Fleischer D E, Mastropietro C.et al Prospective analysis of complications 30 days after outpatient colonoscopy. Gastrointest Endosc 199950322–328. [DOI] [PubMed] [Google Scholar]
- 24.Gow J. Costs of screening for colorectal cancer: an Australian programme. Health Econ 19998531–540. [DOI] [PubMed] [Google Scholar]
- 25.Whynes D K, Nottingham FOB screening trial Cost‐effectiveness of screening for colorectal cancer: evidence from the Nottingham faecal occult blood trial. J Med Screen 20041111–15. [DOI] [PubMed] [Google Scholar]
- 26.Wait S, Schaffer P, Seradour B.et al The cost of breast cancer screening in France. J Radiol 200081799–806. [PubMed] [Google Scholar]
- 27.UK Flexible Sigmoidoscopy Screening Trial Investigators Single flexible sigmoidoscopy screening to prevent colorectal cancer: baseline findings of a UK multicentre randomised trial. Lancet 20023591291–1300. [DOI] [PubMed] [Google Scholar]
- 28.Segnan N, Senore C, Andreoni B.et al Baseline findings of the Italian multicenter randomized controlled trial of “Once‐Only Sigmoidoscopy”‐Score. J Natl Cancer Inst 2002941763–1772. [DOI] [PubMed] [Google Scholar]
- 29.Gondal G, Grotmol T, Hofstad B.et al The Norwegian colorectal cancer prevention (NORCCAP) screening study. Baseline findings and implementations for clinical work‐up in age groups 50‐64 years. Scand J Gastroenterol 200338635–642. [DOI] [PubMed] [Google Scholar]
- 30.Guittet L, Bouvier V, Mariotte N.et al Comparison of a guaiac‐based and an immunochemical fecal occult blood test in screening for colorectal cancer in a general average‐risk population. Gut 200756210–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rasmussen M, Kronborg O, Fenger C.et al Possible advantages and drawbacks of adding flexible sigmoidoscopy to Hemoccult‐II in screening for colorectal cancer. Scand J Gastroenterol 1999173–78. [DOI] [PubMed] [Google Scholar]
- 32.Berry D P, Clarke P, Hardcastle J D.et al Randomized trial of the addition of flexible sigmoidoscopy to faecal occult blood testing for colorectal neoplasia population screening. Br J Surg 1997841274–1276. [PubMed] [Google Scholar]